Australian Waterlife
by Dr. Robert Walsh
September 7, 2022
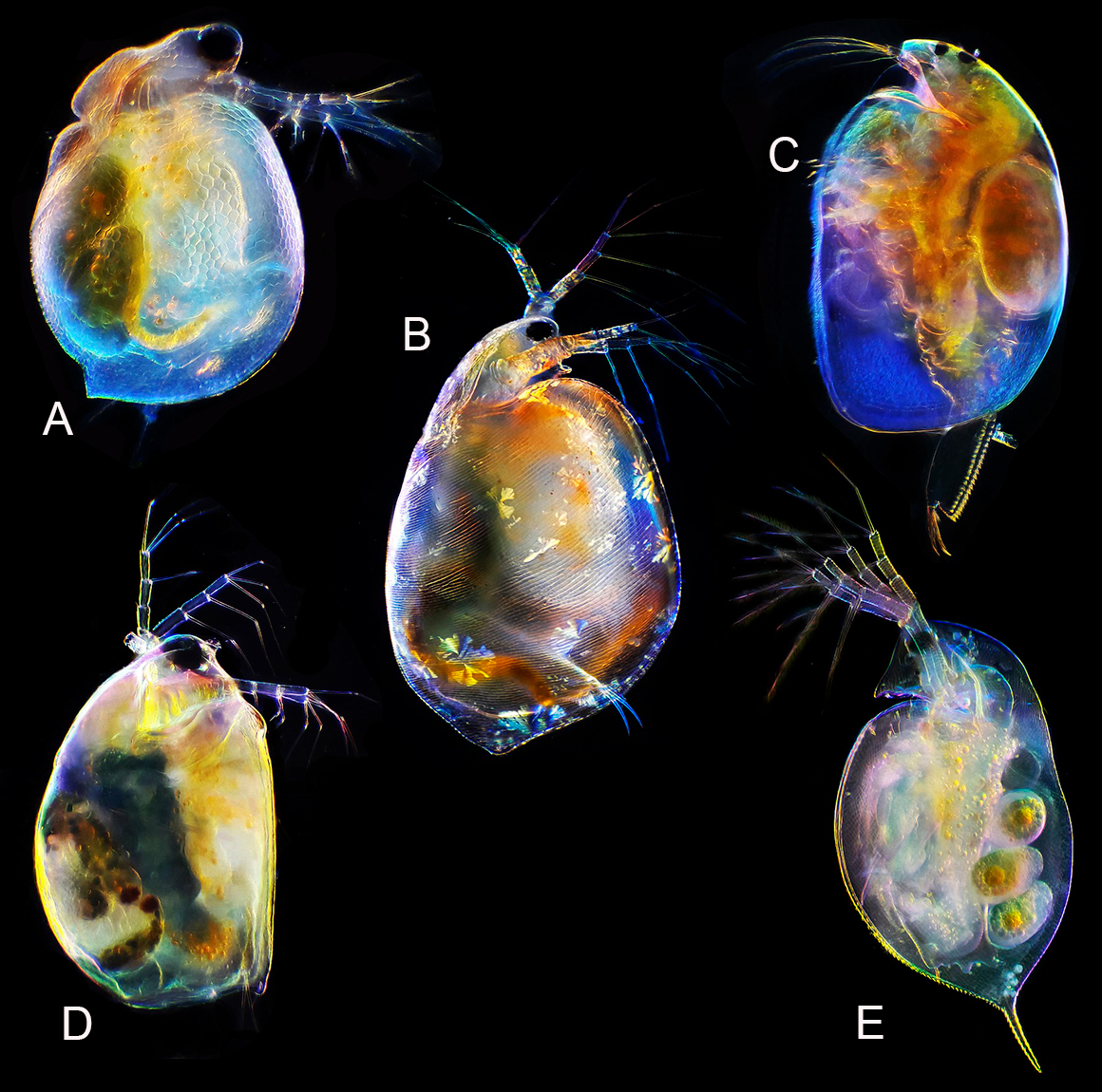
The Diplostraca or Cladocera, commonly known as water fleas, are a superorder of small crustaceans that feed on microscopic chunks of organic matter. Photo by Andrei Savitsky Wikipedia Creative Commons.A. Ceriodaphnia sp. B. Simocephalus sp. C. cf. Aloninae D. Scapholeberis sp. E. Daphnia sp. by dark-field microscopy 50X. cf means compare to or confer.
The Freshwater Microfauna, commonly called zooplankton are typically comprised of four major groups of microscopic invertebrate animals, smaller than 2-3mm: these are the rotifers, ostracods, copepods and cladocerans. The aquatic microfauna are argueably the most important link in the aquatic food web.
Taxonomy is the classification of living organisms and very briefly is the practice of identifying different organisms, classifying them into categories based on shared characteristics, and naming them. See diagram of how living things are classified below.

The major ranks: domain, kingdom, phylum, class, order, family, genus, and species. Diagram modified from Wikipedia Commons by Annina Breen
Phylum: Rotifera
The Rotifera are a microscopic group of small aquatic animals with the majority only 0.05 to 0.2mm (about 50-200µm) in total length. There are 1000µm in 1mm. Therefore 1µm = 1000th of a mm. Thus to see these animals you need a good microscope.
There are an estimated 2300 species known worldwide, with approx. 850 known from Australia spread across 2 taxonomic Classes, and 29 Families. The life cycle is typically only 4-5 days, with eggs may be produced as little as 1 day after the parent itself hatched.

Brachionus sp rotifer with 2 eggs attached. Note the red eye-spot.
Rotifers are extremely important in freshwater environments due to having one of the highest reproductive rate of the microfauna, thus obtaining high population densities in short times, being the dominant animal life in the majority of aquatic microfaunal communities, both in number and diversity.
Rotifers use a variety of methods to obtain their food. In filter or suspension feeding a rotifer consumes tiny algae, bacteria, and protozoa by creating filtering currents that bring small particles to its mouth (e.g., Brachionus, Keratella). Many rotifers that crawl over surfaces feed by a scraping action that removes food from the surface. Some large rotifers such as the planktonic genus Asplanchna and the sessile genera Collotheca feed by grasping and swallowing their prey whole (raptorial). A small number of rotifers are detritus feeders and a few are parasitic. Some consume a variety of foods, while others are extremely specific.
Syncheata sp of Rotifer by phase contrast microscopy 400X.
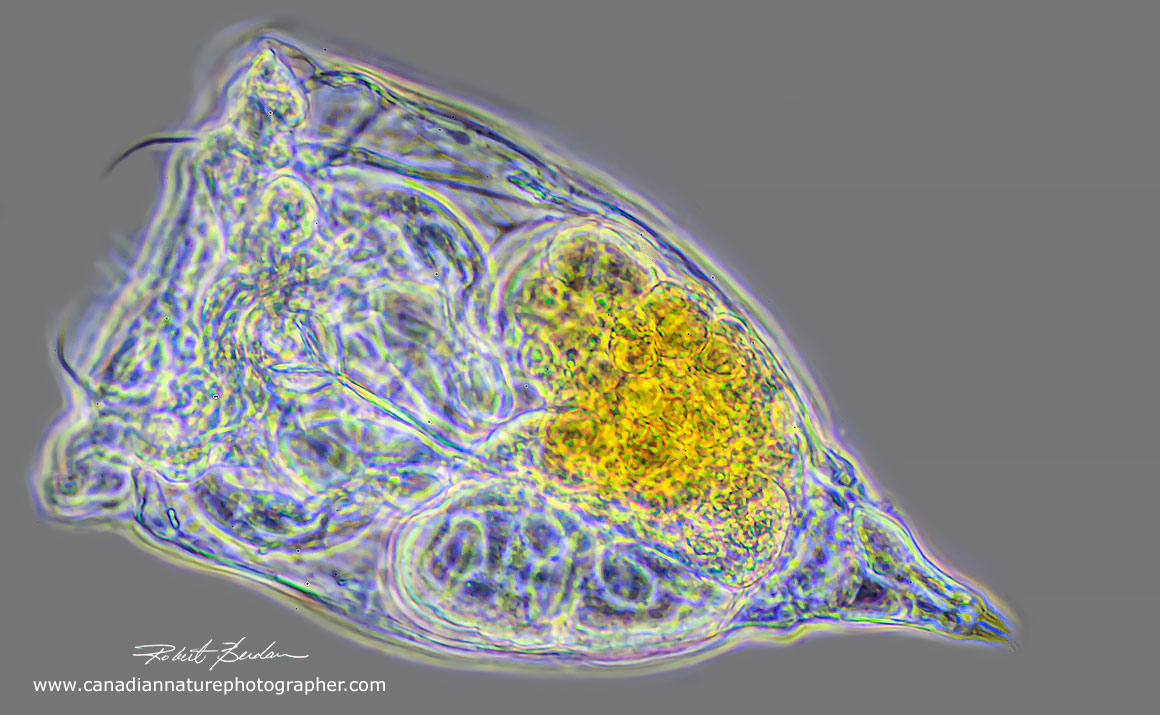
One of the largest rotifers Asplanchnopus multiceps with two chydorine chydoreds in the gut Alonine and Chydorine chydorid. Differential interference microscopy (DIC) 100X.
Rotifers are the most abundant multicelled organisms within inland waters….both numerically and in species number. For example within organically enriched waters of some sewerage and aquaculture ponds, numbers may reach as high as 500,000 individuals per Litre. More commonly rotifer densities are 1-2000 individuals per L. of water
Rotifers have a complex life cycle, and the greater majority consume bacteria, algae, diatoms and protozoa. Depending on the species may take >1-200 algal cells per minute

Some different forms of Rotifera: Rotifer populations are affected by a number of different environmental variables including: temperature, % oxygen, pH, light intensity, food availability and quality, competition and predation to name few.
As rotifers have such short life spans, only for a few days, therefore the rate at which blooms may develop is extremely rapid. Maximum densities are reached within a matter of days.
Also the change in water phys/chem characteristics following a rain event, industrial spill, fire event in the catchment may have a rapid affect on these animals in stormwater wetlands.
These animals though tiny are not to be underestimated. It has been estimated that within wetlands systems, as a group Rotifers may remove up to 72% of the daily production of algae and protozoan biomass.
Class: Copepoda
Copepods constitute a Class which is widespread in both freshwater and marine environments. Whilst there are 10 different taxonomic Orders, the main three Orders that are found within inland waters are the Calanoida, the Cyclopoida and the Harpacticoida.
There are approximately some 13,000 species of copepods known globally, and of these 2,800 of them live in fresh water.
Copepods occupy a variety of different habitats. Some species have a planktonic life style living in the open water environment of lakes, wetlands, reservoirs etc. Others are found in the shallows, often among aquatic vegetation. This area is known as the littoral zone and others are benthic, living on the stream or lake bottom. A number of species may live in moist habitats and swamps, under leaf fall in wet forests, bogs, springs, ephemeral ponds, and puddles, damp moss, or water-filled recesses of plants such as pitcher plants. Species are known to live underground in marine and freshwater caves, sinkholes, or stream beds. Copepods are often used as biodiversity indicators.
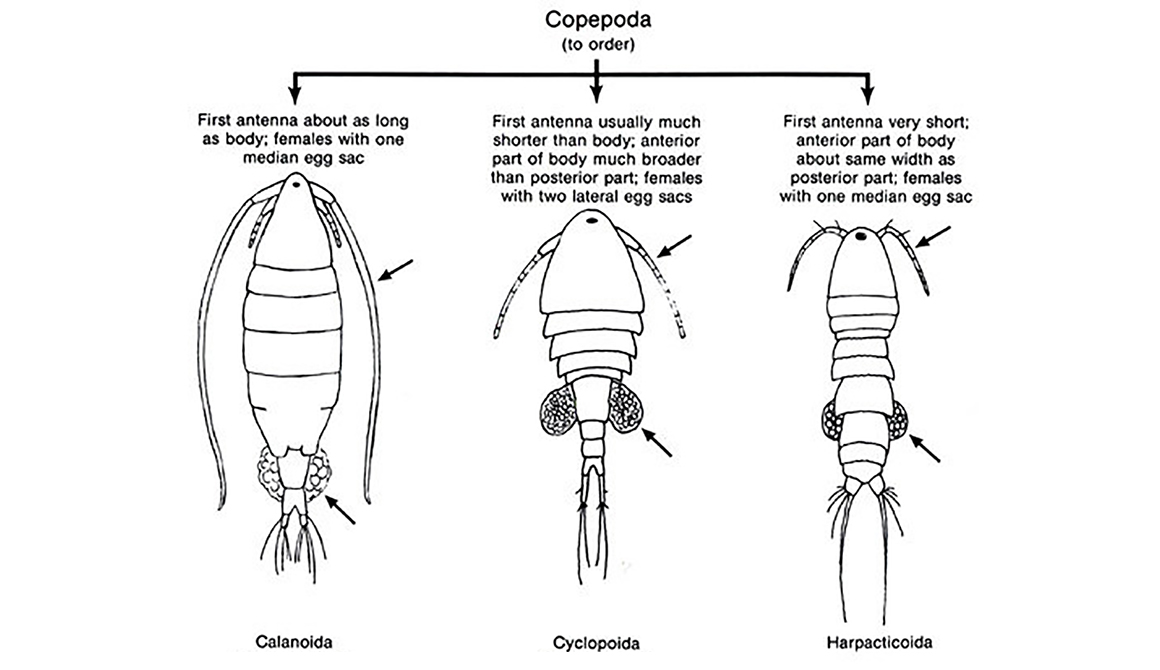
Diagram by Shubhadeep Ghosh - Research Gate
As with other crustaceans, copepods have a larval form. For copepods, the egg hatches into a nauplius form, with a head and a tail but no true thorax or abdomen. The larva moults several times until it resembles the adult and then, after more moults, achieves adult development. The nauplius form is so different from the adult form that it was once thought to be a separate species.

Variety of cyclopoid copepods, some with eggs sacs. Photo by Andrei Savitsky Wikipedia Creative commons using dark-field and polarization microscopy.
With each moult, the body of the nauplius elongates and additional appendages appear. In the copepodite stages which resemble the adult, distinct body segmentation appears, appendages are added, and older appendages develop further.
Copepod nauplius larva dark-field microscopy 200X
The duration of development from egg to adult is governed primarily by temperature. A complete cycle may take three to four weeks at warmer temperatures and up to several months at low temperatures. Some species can be found year-round, others only during the warmer months, and yet others only during late autumn, winter, and early spring. The development rate can also vary depending on the productivity of the waterbody. In addition, growth rates vary among species. As a result, a species may have one to several generations per year in a given body of water.

There are six life stages for copepods after the nauplius stage. Each life stage stage can be defined by the number of legs an individual has. 1st stage = 2 swimming legs, 2nd stage = 3 swimming legs, 3rd stage = 4 swimming legs, 4th stage = 5 swimming legs, 5th stage = pre-adult stage; determined by enlargement of the urosome and development of sexual organs
6th stage = adult stage; 5th leg is fully complete. Diagram from U of C Davis.There are 5-6 Naupliar stages and then there are 5 Copepodite stages with the last stage the mature reproductive adults
Copepod nauplius larva viewed from the side 200X DIC microscopy.
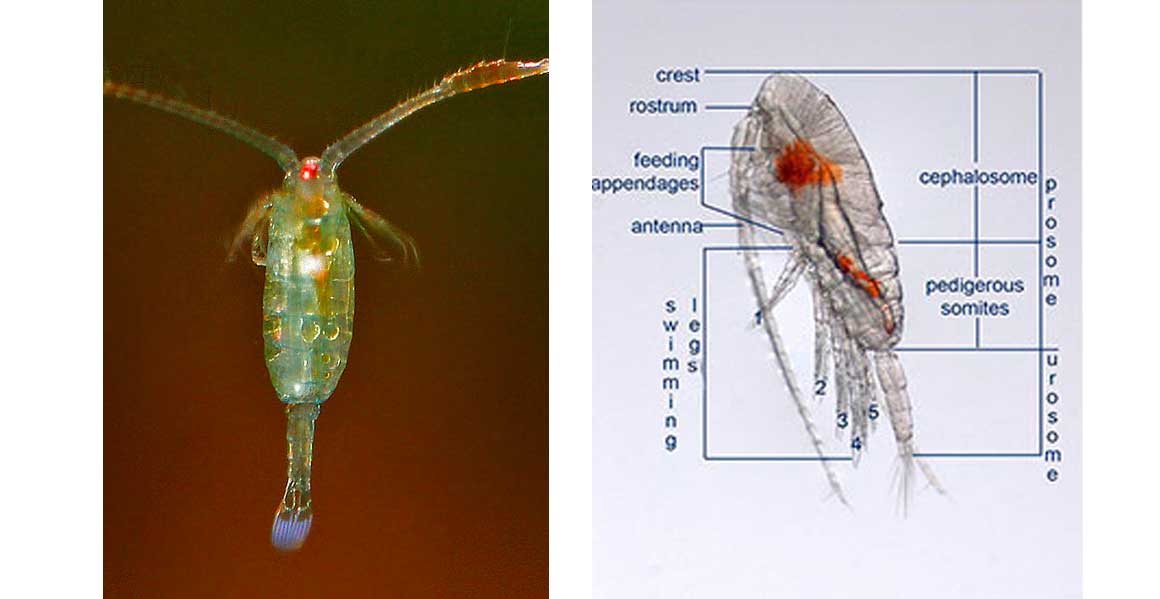
Copepods vary in size considerably, but can typically be 1 to 2 mm long, with a teardrop-shaped body and large antennae. Like other crustaceans, they have a protective exoskeleton, but they are so small that in most species, this thin armour and the entire body is almost totally transparent. Most copepods have a single median compound eye, usually bright red and in the centre of the transparent head; subterranean species may be eyeless. Like other crustaceans, copepods possess two pairs of antennae; the first pair is often long and conspicuous.
Most free-living copepods feed directly on algae, catching cells individually. A single copepod can consume up to 373,000 algal cells per day. They generally have to clear the equivalent to about a million times their own body volume of water every day to cover their nutritional needs. Many of the larger species are predators of their smaller relatives.
Many benthic adult copepods eat organic detritus or the bacteria that grow in it, and their mouth parts are adapted for scraping and biting. Herbivorous copepods, particularly those in rich, cold waters, store up energy from their food as oil droplets while they feed in the spring and summer on plankton blooms. These droplets may take up over half of the volume of their bodies in some species, giving the animal a distinct blue or red colour.

Photo: Thermocyclops sp. by E. Hopp. Used with permission.
Showing the twin egg sacs of a cyclopoid copepod
Planktonic copepods are important to global ecology and the carbon cycle. They are usually the dominant members of the zooplankton, and are major food organisms for small fish and other crustaceans. Some scientists say they form the largest animal biomass on earth.
Because of their small size and relatively fast growth rates, and because they are more evenly distributed throughout the world's oceans, and inland waters, copepods almost certainly contribute far more to the secondary productivity of the world's oceans, and to the global ocean carbon sink, and perhaps more than all other groups of organisms together.
The three Orders of the Copepoda found in inland waters are discussed below.
Class: Copepoda
Order: Calanoida

Calanoid copepod most often found in open water. Dark-field microscopy 100X
In Australian inland waters species belonging to the Calanoida are divided amongst 2 taxonomic Families. The Centropagidae with 4 genera and with 41 species are found across the country. And the Diaptomidae occurs in northern Australia with 1 genus and 2 species. The pictures below are typical of the copepod Centropagidae microfauna (e.g. Boeckella and Calamoecia).
Typical calanoid copepods
These species are found mainly in the open water environment of Australian inland waterways and standing bodies of water, such as reservoirs or lakes. The free-swimming copepods move through the water in jerky motions by moving their swimming legs (refer right diagram).
These small animals typically are herbivorous or omnivorous, feeding on algae, detritus and even bacteria. Typical body length is 700µm-1.7mm. A few species are predatory, opportunistically attacking other copepods, cladocerans and insect larvae.
The taxonomy of the Calanoida is based on the arrangement of the 5th pair of legs in the males of the species (refer photo above). Copepods have a hard exoskeleton, many legs (used for swimming and gathering food), a segmented body, and jointed appendages. Although lacking compound eyes, they have a single simple eye in the middle of the head (sometimes it is only present in the larval stage); this simple eye can only differentiate between light and dark. There are two pairs of antennae; one long and one short.
Calanoids generally eat bacteria, diatoms, and other tiny, single-celled organisms in the water. However some of the larger species here in Australia are quite voracious predators consuming aquatic macroinvertebrate larvae. Others consume rotifers and other small micro-fauna. Calanoid copepods are important in our waters as this group of animals provide a link between the bacterial decomposition pathway and the food web present in our waters. They are a major food source of many macroinvertebrate species, juvenile and small fish species in many freshwater systems.
Class: Copepoda
Order: Cyclopoida
There are currently 27 Genera of Cyclopoids found within Australian inland waters with approximately 120 different species. Each species has their own ecology and environmental preferences. Many are voracious predators with some even noted for attacking fish larvae. As predators cyclopoids may have a substantial impact on their prey, for example Mesocyclops sp. may crop >25% of its prey population per day.

The above two pictures are typical of the cyclopoid microfauna found in many inland waters and associated water ways. These animals are typically found in the littoral zone of inland waters and are quite voracious predators. Some taxa have even been recorded attacking, killing and consuming fish larvae (e.g. Carp, trout, etc.). In one study nearly 90% of the fish larvae (fry) were removed by cyclopoid predation.

Trout Alevin - Larval stage of fish, just hatched from an egg.
Fish fry when first hatch from eggs are immobile due to the attached egg sac. So for the first 1-2 days of their life they are open to predation by cyclopoid copepods.
The taxonomy of the cyclopoids is based on the arrangement of the respective segments of the female 5th pair of legs of the females. See the Pictures below. Typical body length is 600µm -1.3mm.

The above pictures are of the genus Mesocyclops. The level of microscopy required is shown in the second picture of the 5th pair of thoracic legs on which taxonomic identification is based on the arrangement of leg segments.
There is no published key to the Australian Cyclopoida. All taxonomic identification is based on material gleamed from internationally published material.
Class: Copepoda
Order: Cyclopoida
Genus: Macrocyclops sp.
The members of this cyclopoid genus are the largest known from Australian inland waters. The picture below is of the genus Macrocyclops. There are two species of this genus currently identified from Australian waters Macrocyclops albidus and Macrocyclops distinctus.

Macrocyclops sp. Photo E.Hopp. Used with permission.
Their distribution and abundance may be attributed to pH and temperature, but the effects vary with the species. In the benthic regions of lakes it is not uncommon to find copepod densities up to 70,000 individuals per metre squared.
The level of microscopy required on which taxonomic identification of the Cyclopoida is based, is shown in the picture below of the 5th pair of thoracic legs. Differences in the shape and arrangement of leg segments allow identification of species.

Photo: The 5th leg of a female Macrocyclops sp.
Macrocyclops spp. are quite large when fully mature, with reproductive adults 1.6-2.1mm in total body length. Specimens have been known to reach 3-4mm.This genus occurs largely within the littoral and benthic zone of ponds, wetlands etc. It is a voracious predator attacking not only rotifers, cladocerans, copepods but also the early stages of its own species, plus the larval stage of aquatic insects.
Copepods have been used successfully in Vietnam to control disease-bearing mosquitoes. The copepods can be added to water-storage containers where the mosquitoes breed. Copepods, primarily of the genera Mesocyclops, Thermocyclops and Australocyclops, eat the younger first- and second-instar larvae stage of the mosquitoes.
Trials using copepods to control container-breeding mosquitoes are underway in several other countries, including Thailand and the southern United States
Such predatory cyclopoids are also believed to be a primary factor in controlling the distribution and abundance of Rotifera and larval aquatic macroinvertebrates.
Class: Copepoda
Order: Harpacticioda
The taxonomic Order Harpacticoida comprises 463 genera and about 3,600 species worldwide; its members are benthic copepods found throughout the world in the marine environment and in fresh water. A few of them are planktonic in life style.
Harpacticoids are distinguished from other copepods by the presence of a very short pair of first antennae. The second pair of antennae are biramous. They typically have a wide abdomen, and often have a somewhat worm-like body.

Photo by R. Berdan www.canadiannaturephotographer.com
The Harpacticoida are a mainly free-living benthic aquatic organisms. The greater majority of those found in freshwaters are benthic or interstitial, that is living between the grains of sand and gravel within inland waterways, although many species have successfully exploited other habitats, including semi-terrestrial habitats. They have been found living within pitcher plants, and the moss prevalent in the temperate rainforests of Tasmania, a large island off the south-eastern Australian mainland.
Harpacticoids have a size range between 0.2-2.0 mm. But the greater majority are small, often less than 500µm. Their body morphology is characterized by a body formed by several articulated segments that form two separate regions; the anterior prosome and the posterior urosome.
The adults retain the central eye of the larval stages, with the exception of some underground
species that lack visual organs. The harpacticoids have shorter first antennae, and a relatively
wider urosome (refer diagram) than the copepods from other orders. The basic body plan of harpacticoids is more adapted to life in the benthic environment than in the open water (pelagic) environment.
A harpacticoid copepod begins life as a fertilized egg, which then hatches into a nauplius larva. The nauplius has six stages. With each moult, the body of the nauplius elongates and additional appendages appear. In the copepodite stages which resemble the adult, distinct body segmentation appears, appendages are added, and older appendages develop further.
The duration of development from egg to adult is governed primarily by temperature. A complete cycle may take three to four weeks at warmer temperatures and up to several months at low temperatures. Some species can be found year-round, others only during the warmer months, and yet others only during late autumn, winter, and early spring. The development rate can also vary depending on the productivity of the waterbody.
In addition, growth rates vary among species. As a result, a species may have one to several generations per year in a given body of water.
Under unfavorable conditions, harpacticoids may produce resting eggs or encyst as adults to bridge the gap between seasons or periods of drought. The cyst encasing an adult is a hollow sphere of silt and detritus material glued together with secretions. The main function that encysting serves is to protect individuals from prolonged dehydration.
In addition to improving a copepods chance of survival under adverse conditions in its home body of water, these resting stages provide a means for the transfer of species from one locality to another, by wind, water or passive transport on waterfowl, animals or even humans.
Harpacticoid copepods occur in a variety of aquatic and semi-aquatic habitats. In standing or flowing water, they may be found in amongst aquatic plants, detritus, or bottom sediments. They also may be found in interstitial sands or shoreline debris of streams, rivers, lakes, springs, and seeps; sometimes up to several meters above the shoreline. In general, they are found to a depth of 40m in some lakes, with the highest abundances found in shallow areas of inland waters.
Harpacticoids are primarily surface-feeders that use their mouthparts for scraping or seizing food from a variety of substrates. They may consume algae, fungi, protozoa, bacteria, and detritus. Carnivory has been reported in harpacticoids, but predation is not thought to be an important method for obtaining food in most species.
Harpacticoids are very important as primary and secondary consumers and are a major food source for both larger invertebrates and many small or juvenile fish species. Researchers have found that stream harpacticoids consumed up to 22% of the daily carbon production.
Harpacticoid populations may double in size in less than two weeks, under optimal food and temperature conditions. It is not uncommon to find densities of harpacticoids ranging from 30,000-40,000/m2 in lake systems of the northern hemisphere.
The Harpacticoids are a very diverse group of copepods both in terms of morphological diversity and in the species-richness of some of the families. However because of their small size and habitat, they are frequently overlooked in ecological studies.
Class: Branchiopoda
Order: Cladocera :

A cladoceran giving birth (100X). Photo by Marek Mis using Darkfield and Polarized light. Photo from Wikipedia creative commons.
Cladocerans other-wise known as water fleas are tiny, often transparent animals, whose general shape and jerky swimming motion accounts for their common name of “water fleas”. Although abundant in nearly all inland waters they are often overlooked.
A total of eight Families of Cladocera are known from Australian inland waters. These are the Sididae, Bosminidae, Chydoridae, Daphniidae, Ilyocryptidae, Macrothricidae, Gondwanothricidae and Moinidae. The more common cladocera encountered within Australian waters are discussed below.
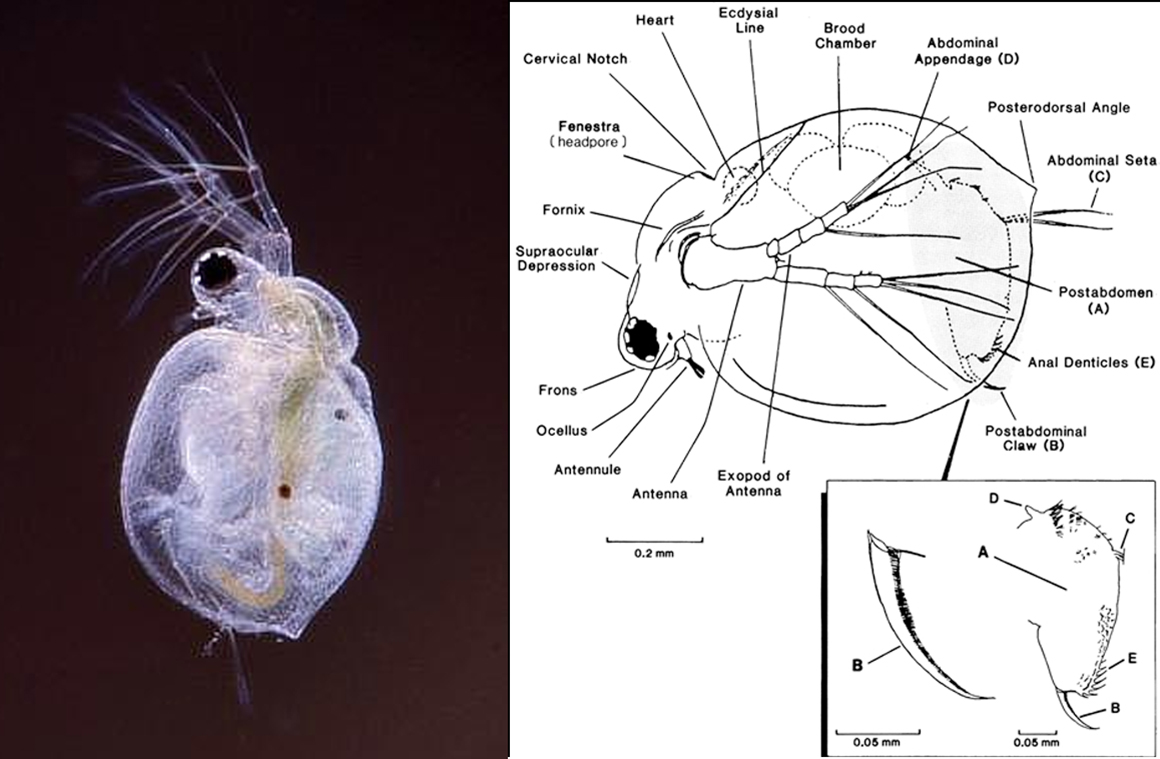
Generally cladocerans are small in size with the greater majority of species being less than 1mm in total length with a head and single eye, plus a carapace that covers the animal’s body (thorax and abdomen). The head may be separated from the rest of the body by a "cervical sinus" or notch. It bears a single eye, located on the animal's midline, and often, a single ocellus is present. The ocellus is a photoreceptive device and is used by the animal to tell which way is up, that is where the sun is.

Photo: Daphnia sp. R.Berdan. The 2nd Photo are the filtering combs of Daphnia viewed with a scanning electron microscope. To give some idea of size, the white line (scale bar) at the bottom of the photo is 10µm in length.
The head also bears two pairs of antennae – the first antennae are small, unsegmented appendages, while the second antennae are large, segmented, and branched, with powerful muscles. The first antennae bear olfactory sensors, largely used to detect chemical signals in the water regarding food, while the second pair are used for swimming by most species. The pattern of setae on the second antennae is also useful for identification of the animal. The part of the head which projects in front of the first antennae is known as the rostrum or "beak". The mouthparts are small and used to eat algae, organic detritus of all kinds and bacteria.
Cladocerans have five or six pairs of delicate leaf-like appendages (legs) surrounded by the carapace for protection, each with numerous hairs or setae, that act like a mesh to capture small particles. In Daphnia there are 6 of these filtering legs. These “filtering combs” beat the water creating feeding currents, and in turn these setae are used to filter the water and capture algae, detritus or bacteria. This food is then transferred to the mouth by long bristles on the legs acting to sweep the caught particles towards the mouth. The cladocera don’t have lungs like we do. Instead carbon dioxide is lost, and oxygen taken up, through the body surface.
Shiel and Ganf 1985 noted that in Australia, Daphnia were able to selectively increase their consumption of smaller algal/bacteria cells <4µm, than larger <9µm. Hence the Daphnia - bacteria trophic link should be considered to have significant ecological implications in freshwaters.
In some species groups the foot is used for identification of the animal. The shape of the foot, presence or absence of a claw, the no of spines or denticles (tiny teeth) or setae; may all tell us which species the animal may be.
Cladocerans produce two types of eggs. The lifecycle of cladocerans is dominated by asexual reproduction (parthenogenesis), with occasional periods of sexual reproduction: When conditions are favourable, reproduction occurs by parthenogenesis, producing only cladoceran female clones, which then give rise to more female clones.
A female Cladoceran produces a clutch of parthenogenetic eggs after every adult molt (if feeding conditions permit). The eggs are placed in the brood chamber, which is located dorsally beneath the carapace.
With the onset of the Australian summer, conditions in the water body (wetland, lake, reservoir, temporary water body) deteriorate usually by the water body drying up. This acts to increase the conductivity of the water. The cladoceran female clones are stimulated to produce male offspring, and following this, sexual reproduction occurs. This results in the production of long-lasting “resting” eggs encased in an ephippium. These dormant eggs sink to the sediments and hatch when environmental conditions are favourable. These “resting eggs” are resistant to drying out, and may also be dispersed by wind from the dried out wetlands etc, and hatch when they reach favourable conditions, allowing many cladoceran species to have very wide distributions.
Nearly all cladoceran species live in fresh water and other inland water bodies, with only eight species being marine. They display a very wide range of feeding types and have adapted to many ecological niches. Some cladocerans inhabit leaf litter in water, or the sediment surface, others the stems of aquatic plants where they consume a thin film of algae which covers the surface of these plants. Others are planktonic which live in the open water.
Cladocera feed on detritus, bacteria, algae, diatoms, protozoa, and ciliates. In turn these animals provide a food source for the aquatic macroinvertebrates, small and juvenile stages of fish species, and even some birds. These tiny animals link everything in aquatic food webs together, and if this faunal group is missing for any reason, then the system will quickly collapse. To put this simply, No microfauna = no Fish (trout, cod, etc). Therefore managers of wetlands, waterways, reservoirs, water and sewerage treatment plants, need an understanding of the environmental baseline conditions and ecology of these waters that influence these animals.
If not monitored properly these waters (lakes, ponds, reservoirs, wetlands, etc) have the danger of being nothing more than endpoints for drains. For example blue green algae events. The microfauna are a really fundamental link in the ecology of these systems and play a vital role in keeping algal blooms in check as this is a major component of many different animal diets.
Cladocerans occur where ever there may be water; such as lakes, marshes, ponds, dams, pools, puddles, temporary waters, billabongs, cut-off river meanders, ox-bow lakes, wetlands, and even in sphagnum moss. Most species are sensitive to salinity.
Typically the cladocerans are the most abundant of the micro-crustaceans within aquatic systems, reaching densities greater than 1 million animals per metre squared, with as many as 30 species being present.
Whether a species occurs in a body of water depends upon:
1. Probability of colonization of the water body
2. Chemical and physical requirements of the species
3. Food conditions within the water
4. Predators living within the waer
Almost all members of the Families Chydoridae, Ilyocryptidae and the Macothricidae are benthic, or living on and in various surfaces, particularly aquatic macrophytes, coarse plant debris, other organic debris and swim only very short distances.
Most species of the remaining Families of cladocerans are primarily planktonic where their swimming lifestyle makes them independent of surfaces. Besides differing in habitat, planktonic and benthic cladocerans differ in ecological relationships. Therefore to say that everything learnt about Daphnia or Chydorus applies equally to all cladocerans is misleading and incorrect.
Order: Cladocera:
Family: Daphniidae:
Genus: Daphnia sp
Daphnia is native to Australia, and inhabit large lakes as well as small temporary pools, and is found in the littoral zone of many inland waters, and often in the open waters of impoundments and wetlands.
Daphnia may be the largest of the aquatic microfauna, with individuals reaching 5mm in total body length. They are filter feeders and ingest unicellular alga, protozoa and bacteria
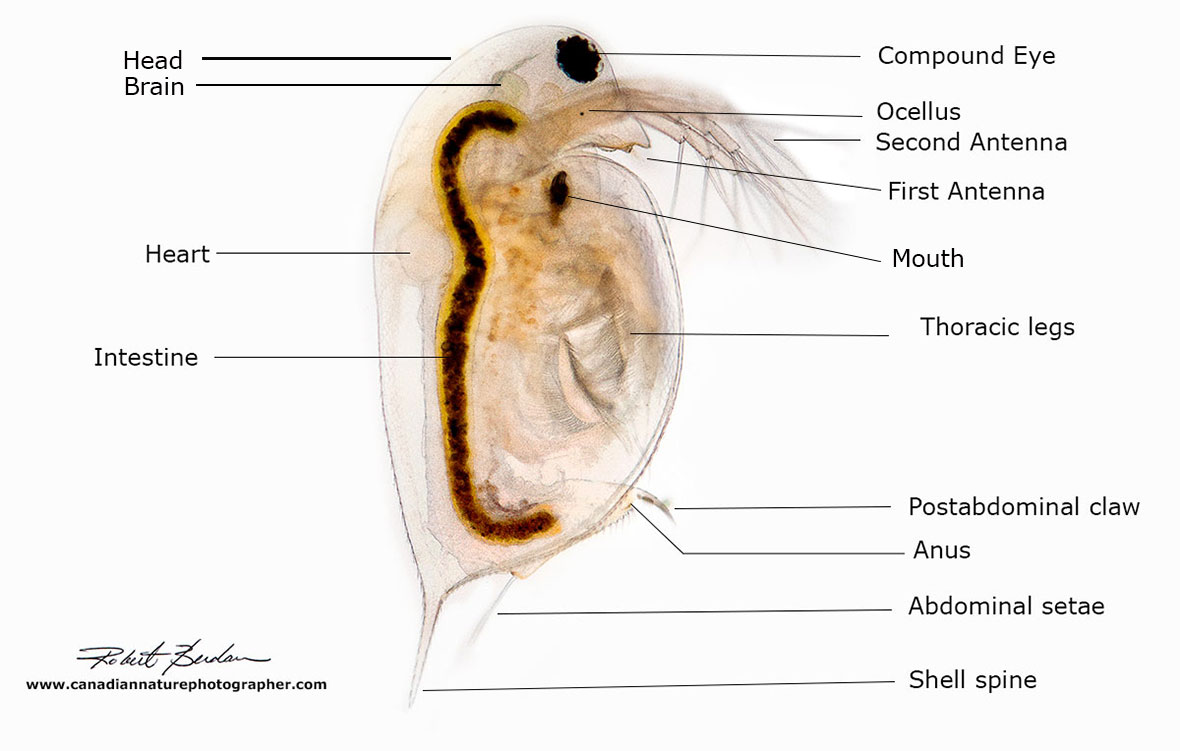
Labelled photo of a Daphnia sp. 50X bright-field microscopy
The colour of Daphnia reflects the food that is predominant in their diet. Daphnia feeding on green algae will be transparent with a tint of green or yellow, whereas those feeding on bacteria will be white or salmon-pink. Also well-fed animals are more strongly colored than starved animals.
Similarly low oxygen levels also affect the colour of Daphnia, low oxygen levels in the water the body usually colouring the animals reddish. To support oxygen transport, Daphnia have the extracellular respiratory protein hemoglobin. Daphnia tend to develop more hemoglobin to increase oxygen uptake from the water. In response to environmental changes (oxygen concentration, temperature), the hemoglobin concentration varies up to about 20-fold. This gives the transparent animals a reddish appearance, particularly in waters with low oxygen concentrations.
A filter mesh make the Daphnia a very efficient filter feeder of bacteria and small algae species in freshwaters. The size of the mesh will vary according to the individual species. In a paper by Nagata 1985 he analysed the mesh size of daphnia and filtering rates of natural bacteria. For example Daphnia longispina has a filter mesh of measuring between to 0.17µm-0.36µm. Daphnia hylaena has a filter mesh of measuring between 0.56 -1.8µm. Daphnia galeata has a filter mesh of measuring between 0.32-1.0µm. He concluded that many Daphnia sp are highly efficient bacteria feeders.
A female Daphnia produces a clutch of parthenogenetic eggs after every adult molt (if feeding conditions permit). The eggs are placed in the brood chamber, which is located dorsally beneath the carapace.
At 20ºC, the embryos hatch from the eggs after about 1 day but remain in the brood chamber for further development. After about 3 days in the brood chamber, the young Daphnia are released by the mother. The newborn look more or less like the adult Daphnia, except that the brood chamber is not yet developed. In most species, a juvenile Daphnia passes through four to six juvenile instars before it becomes an adult and produces eggs for the first time. The age at which the first eggs are deposited into the brood chamber is around 5-10 days at 20ºC, but this may take longer under poor feeding conditions. An adult female Daphnia may live for more than 2 months may produce a clutch of eggs every 3 to 4 days until her death, with a higher age being reached under poorer feeding conditions. Clutch sizes vary among species, from 1 to 2 eggs in small species to more than 100 in large species of Daphnia.
This genus has some 14 species and is quite widespread within Australian inland waters. When environmental conditions are right the genus may be present in huge numbers. It is often the taxa present in waterways when organic enrichment, i.e. algal blooms are present, with the population of a water body responding rapidly to algae blooms.
Large planktonic species of cladocerans such as Daphnia appear to be most efficient in cleaning up water bodies made turbid by blue-green algae. The size and feeding rates of large Daphnia make them especially efficient at the clearing of algae from water bodies. It has been reported that a single large Daphnia may remove up to1200 algal cells, 1-50µm in size, per hour. Smaller cladocerans while less prone to predation by fish, invertebrates etc, are less efficient at clearing of algae from water bodies.
In the northern hemisphere this genus is often used in ecological and ecotoxicology studies. More is known about ecology of this particular genus than of all the other genera found in the Cladocera.
Order: Cladocera:
Family: Daphniidae
Genus: Simocephalus sp.
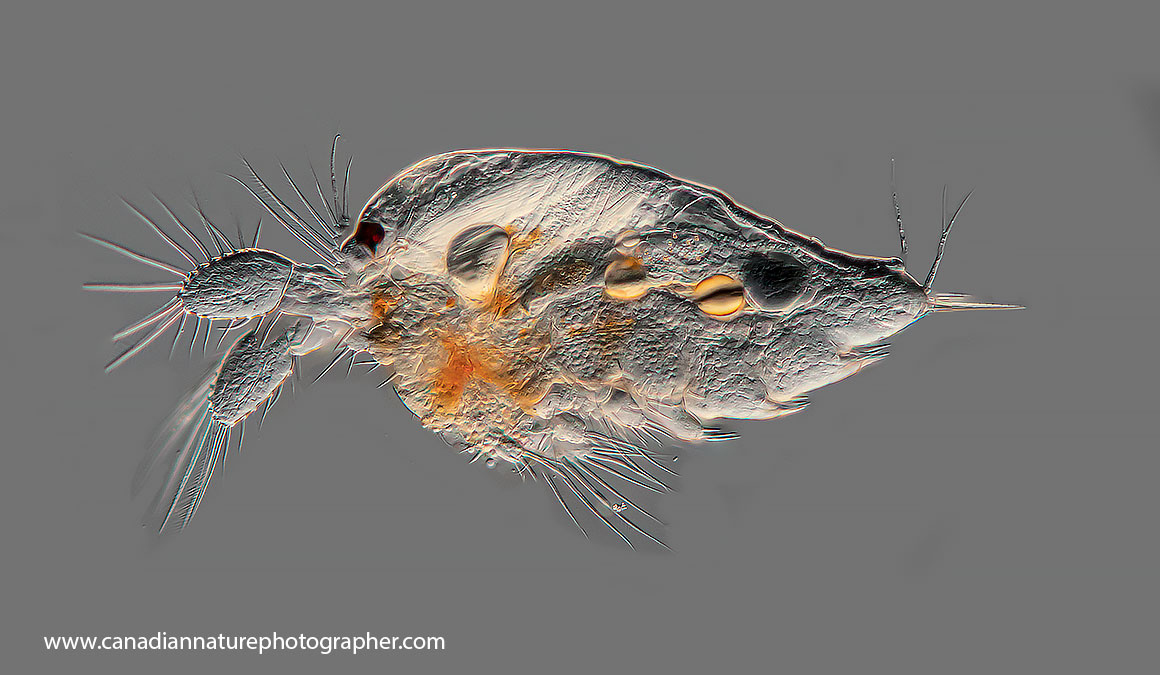
Simocephalus sp. are large aquatic microfauna, 3-4 mm in length, round animals that are covered in a bivalve carapace. The head, which contains a single compound eye, is not covered by this carapace but instead is surrounded by a hood. Attached to the head are a very small rostrum and the first antennae which bear olfactory setae, largely used to detect chemical signals in the water regarding food, while the second pair are used for swimming appendages by most species. The mouthparts of Simocephalus sp. are small. Simocephalus sp. has five thoracic legs used for filtering food or respiration. The dorsal side of the thorax is called the brood pouch, an extension of the carapace, and is where the eggs are held.

Simocephalus sp. Notice the long second antenna. These appendages are used to move Simocephalus through the water. Notice the digestive track, running down the middle of the animal, which is darkly pigmented due to the presence of food.
One of the most important features in differentiating between species is the shape of the ocellus, and post abdominal claw on the foot of the animal. The difference in fine hair like structures (pectin) on these claws is what that separates many species from one another.
Simocephalus sp. is found in what are known as standing bodies of water all over the world, such as wetlands, ponds and temporary waters. There are 7 species known from Australia, typically being found in amongst heavy vegetation in the littoral zone of large shallow wetlands and ponds. However, since these species live in vegetated areas they are vulnerable to habitat destruction by fish species, particularly carp; and farm livestock such as cattle grazing on aquatic plants.
Simocephalus sp. are filter feeders that ingest any algae, protozoan, or organic detritus of the right size. The thoracic legs establish feeding currents, which bring suspended particles through the opening in the bivalve carapace. The setae on these legs remove undesirable particles from the feeding current. Once the filtered food enters the carapace it is directed into the mouth by the very short first antennules.
Simocephalus produce eggs primarily by parthenogenesis (without fertilization). The lifecycle of cladocerans is dominated by asexual reproduction (parthenogenesis) producing only female clones, which then give rise to more female clones.
With the onset of the summer, conditions in the water body (wetland, lake, reservoir, temporary water body) deteriorate, the cladoceran female clones are stimulated to produce male offspring, and following this, sexual reproduction occurs. Female animals then reproduce a sexually producing “resting egg” that lay dormant through the winter and hatches in the spring.
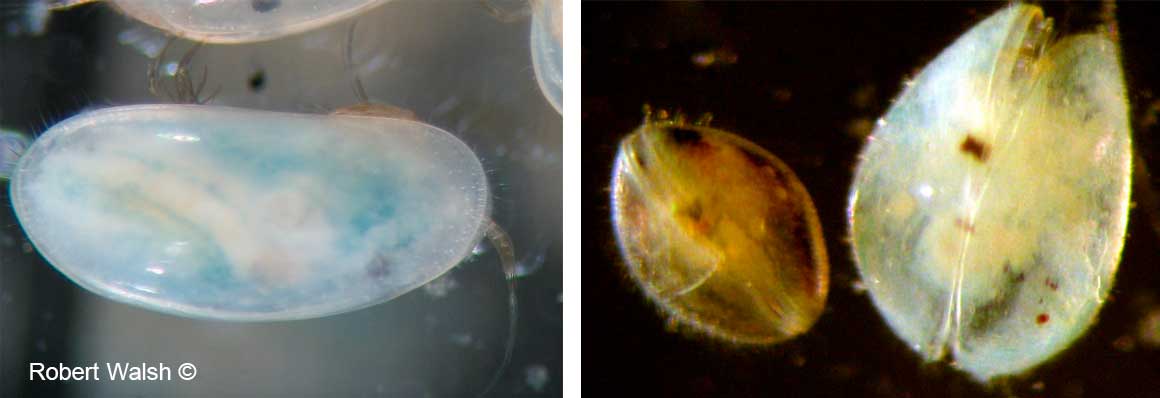
The resting egg is encapsulated in a protective structure called an ephippium and can contain the egg or eggs. The ephippium is cast off on the next molt (see photo below). These resting eggs can survive dry summers, desiccation and ultraviolet radiation. Females are hatched from resting eggs which in turn produce more parthenogenetic eggs. And thus the cycle continues.
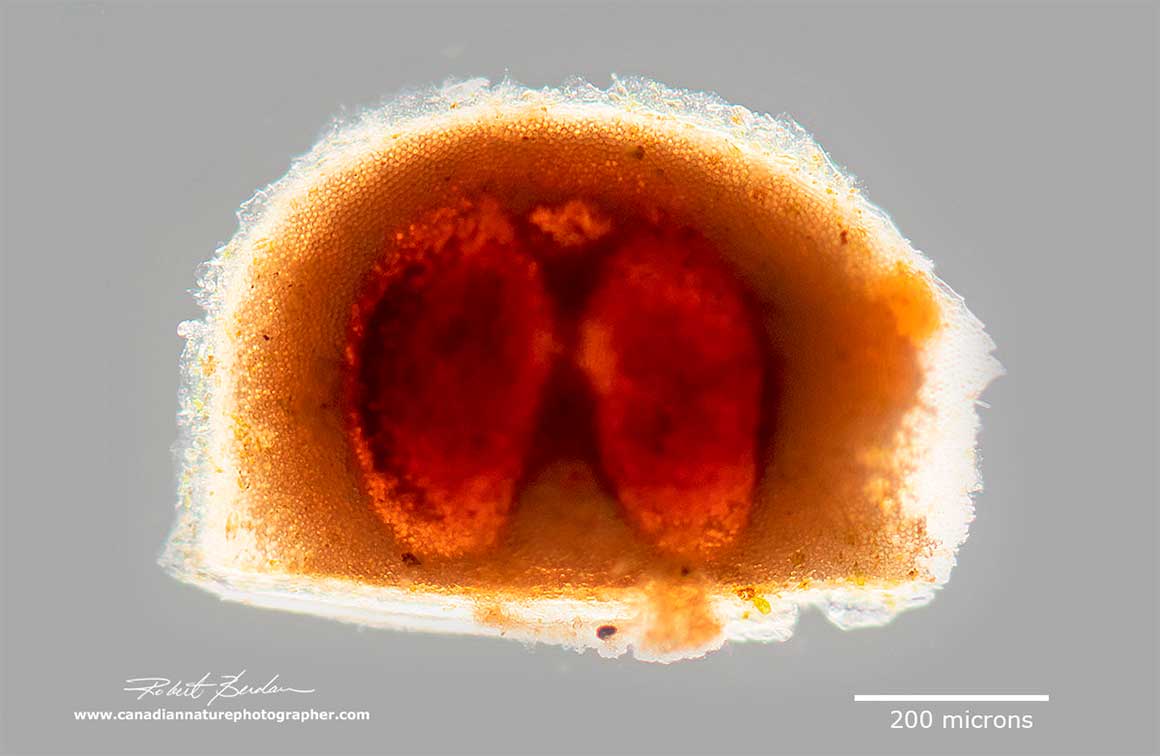
.The picture above is an ephippium containing two eggs from a Daphnia sp. The Simocephalus ephippium contains only a single egg.
Young Simocephalus sp. develop directly into adults with no free swimming naupliar stage. The juveniles go through approximately five moults before becoming sexually mature at nine days. After this point the adults still moult in order to grow, but almost all of their energy goes into reproduction.
Order: Cladocera
Family: Daphniidae
Genus: Ceriodaphnia
Ceriodaphnia species are widespread throughout Australian inland waters. Once believed to be mono-specific, this is now known not to be the case, with up to a dozen species believed to exist within Australia.
Ceriodaphnia sp. Photo E. Hopp. Used with permission.
Species determination within Australia is problematic as the majority of species recorded from Australia are believed to be new, and there is at this stage, no scientific published taxonomic key to the species of this Genus.
The diet of the taxa is believed to be algae. The maximum size of the genus is no more than 0.7mm with most individuals in the range 400-550µm (0.4- 0.55mm).
Ceriodaphnia is often encountered in samples from the littoral zone of water bodies. It may also be encountered within the open waters of large bodies of water if there are no or few predators, such as macroinvertebrates or small fish.
Members of this genus are used for ecotoxicology studies on freshwater catchments by several laboratories around the country. They measure the responses of these animals to toxic substances that may enter freshwater systems.
Order: Cladocera:
Family: Chydoridae :
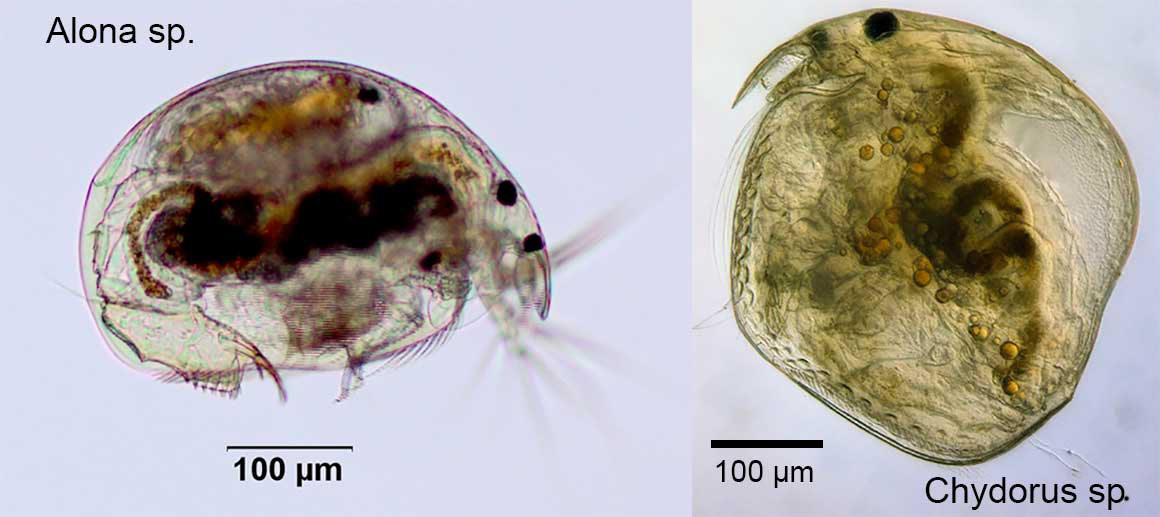
The Chydoridae are the most diverse cladoceran family within Australia and appears to have undergone adaptive radiation to suite environmental conditions. There are more than 30 different genera with more than 82 different species found across the country, with many endemic to particular regions. Australia has the largest number of species and highest level of endemicity in the world.
The Chydoridae occur predominantly in the littoral region of vegetated water bodies in slow moving waterways, lakes, wetlands, billabongs, springs, temporary waters, and water holes throughout the country. They are also present in caves, underground waters and in amongst moist conditions of mosses.
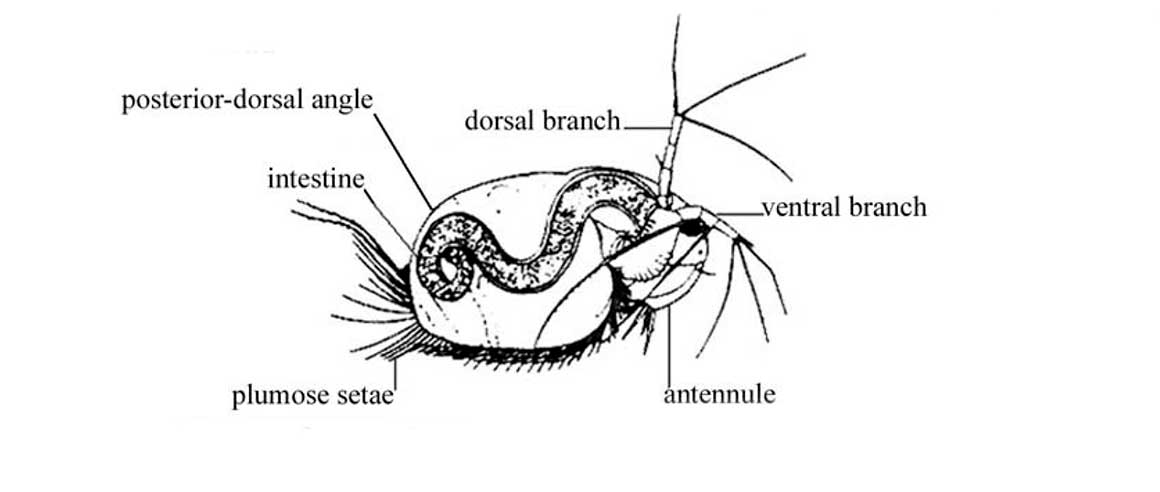
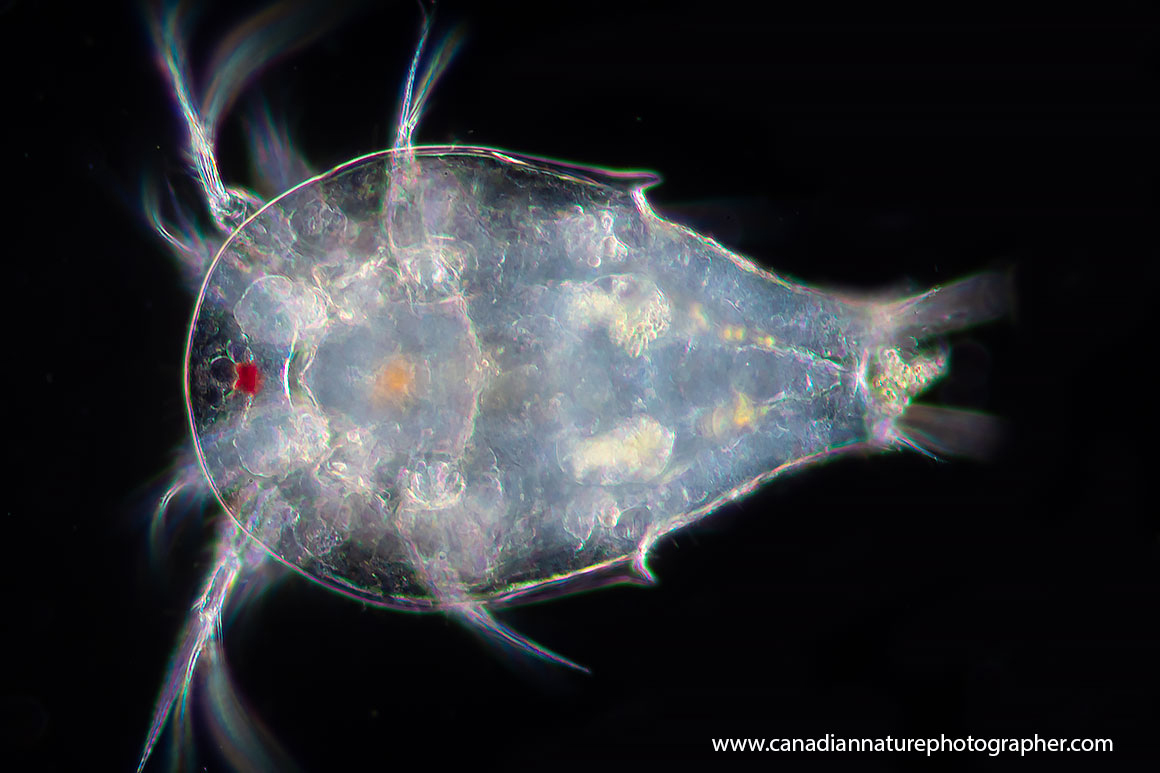
On the left photo note the post abdominal foot: the shape of the indent at base of the tooth, the large spine at the base of the tooth, and the teeth on the foot, are often used to tell species apart.
Some members of the Chydoridae live on the stems or stalks of aquatic vegetation, consuming the film of algal mucilage or Diatoms that may cover such plants. Others live within or just above the sediment, grazing on bacteria and/or detritus.
The Chydoridae typically feed by crawling along surfaces or through the sediment where they scrape up or filter food. Between species there is great variation in diet. Most of the specialization of the Chydoridae seems to be in obtaining food from slightly different microhabitats.
The greater majority of the Chydoridae are usually in the range of 300-600µm, with few individuals or species larger than 700µm (0.7mm) in total body length.
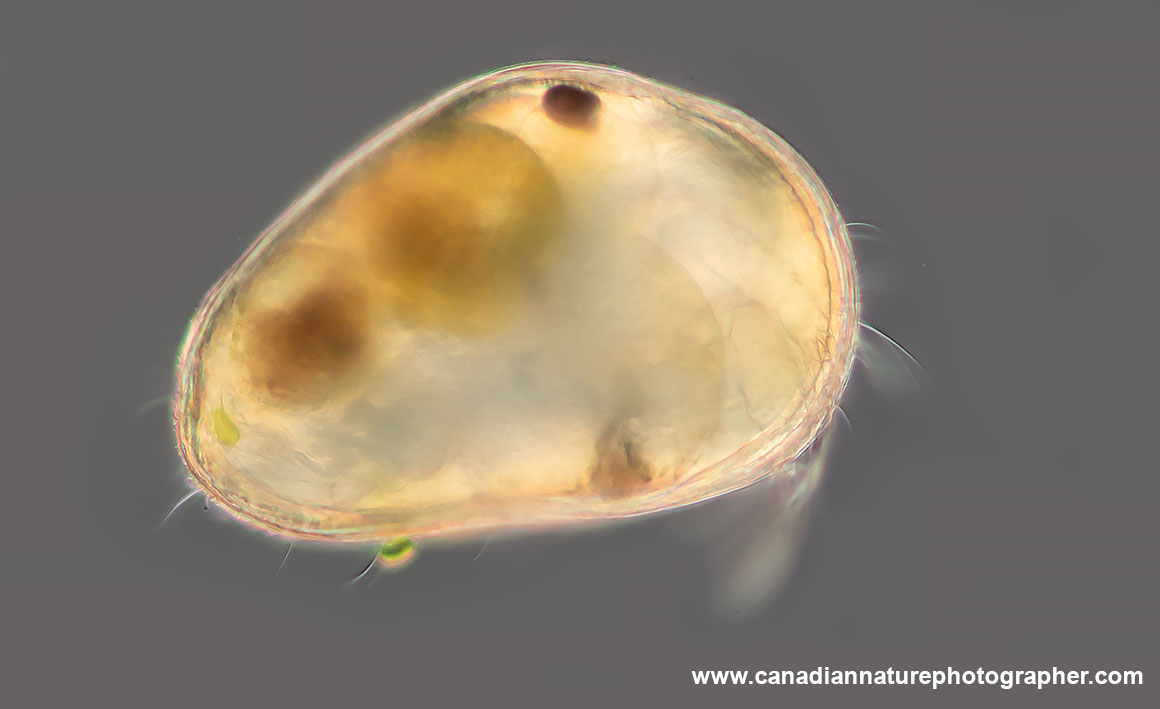
Cladoceran significance in aquatic food webs has largely been ignored in Australia. This is particularly true of the Chydoridae. Firstly taxonomic keys are often inaccessible, incomplete or inaccurate to species level for the average aquatic ecologist. Even at the Family level of taxonomic resolution, the Chydoridae have been confused with members of other Families (e.g. Bosminidae). Secondly as they commonly live in amongst aquatic vegetation and the benthic region of inland waters, they do not appear in plankton samples, the habitat typically sampled for aquatic micro-invertebrates; or their numbers are so low that they are regarded as unimportant. While aquatic vegetation and the benthos is routinely sampled for macro-invertebrates, the chydorids are ignored, or excluded as the mesh size of the net (500µm) misses the majority of species; and as the sampling is for aquatic macro-invertebrates, any chydorids caught are regarded as contaminants, insignificant due to their small size, or under sampled, not identified or counted. However overseas studies show that they have high species richness and high densities, often being the most abundant invertebrate taxa present.
In Europe and the USA, where chydorid taxonomy and ecology is better known, chydorids are used as bio-indicators in studies of change in environmental conditions within water bodies. Chydorids are just as sensitive to environmental conditions as Diatoms, and studies have shown that species composition of chydorid communities is dependent on the physicochemical conditions of a water body. Their body parts preserve well in sediments plus their ecology and community relationships are stable over time, thus making them useful for paleolimnological studies.
Chydorid have radiated extensively within Australia, thus making them good bio-indicators, as in a healthy water body, several species may co-exist in the same environment. Of all the cladocerans they possess the greatest degree of radiation, and in Australian inland waters the levels of selective pressures which may cause genetic divergence seem higher than elsewhere. This makes the Chydoridae ideal for studies on evolution and adaptive radiation.
Order: Cladocera:
Family: Moniidae
Genus: Moina spMoina is a genus of crustaceans within the family Moinidae. The genus was first described by W. Baird in 1850. They are referred to as water fleas but are smaller than their more well-known cousins: the larger Daphnia and Simocephalus. This genus demonstrates the ability to survive in waters containing low oxygen levels as well as high salinity and other impurities, including salt pans, and commonly eutrophication.
Photo. Moina sp E. Hopp. Used with permission.
Many of the biological characteristics that have already been discussed for Daphnia can be applied to Moina.
Moina thrives in ponds and reservoirs but primarily inhabits temporary ponds, wetlands or ditches. The period to reach reproductive maturity takes four to five days at 26°C. At maturity clear sexual dimorphic characteristics can be observed in the size of the animals and the antennule morphology. Males (0.6-0.9 mm) are smaller than females (1.0-1.5 mm) and have long graspers which are used for holding the female during copulation. Sexually mature females carry only two eggs enclosed in an ephippium which is part of the dorsal exoskeleton.
Moina is of a smaller size than Daphnia, with a higher protein content, and of comparable economic value. Produced in high numbers the species is successfully used as feed in the culture of rainbow trout, salmon, striped bass larvae and also by tropical fish hobbyists, who also use this genus to feed over sixty fresh and salt water fish varieties.
Order: Cladocera :
Family: MacrothricidaeThe Family Macrothricidae is one of the least studied groups of microcrustacea found within the littoral zone of inland waters. The Family has a global distribution and are believed to be the most primitive of the Cladocera. They typically are abundant in tropical and subtropical water bodies. Representatives of this family are often found in the temperate waters of southern Australia.
The Macrothricidae occur predominantly in the benthic littoral region of vegetated water bodies in slow moving waterways, lakes, wetlands, billabongs, springs, temporary water, and water holes throughout the country.
General diagram of a member of the family Macrothricidae from - Practical Guide to Identify Freshwater Crustacean Zooplankton - Cooperative Freshwater Ecology Unit 2002, 2nd Edition - North America.
There are 4 Genera within this Family, with approx 17 species belonging to the Genus Macrothrix found in Australian fresh waters. A number of these are endemic to Australia.
Macrothrix is a benthic dwelling genus, found in the littoral shallows of rivers, ponds, wetlands and lakes often amongst aquatic vegetation. Body size is often 0.4-0.8mm. They form an important part of the diet of small adult fish species and juvenile stages of larger freshwater fish.
Class: Ostracoda
Ostracods are small crustaceans and are surrounded by a calcareous shell that has been secreted around them. They are quite widespread though out Australia and are found in all sorts of inland waters. They range in size from 0.4mm up to 5mm.
Candonocypris sp. and Cypretta spp photographed with a stereomicroscope by Robert Walsh.
Ostracods are the oldest known microfauna with fossils dating back to the Cambrian. The ostracods occupy a wide range of habitats and are found in nearly every sort of aquatic habitat. These range from temporary waters to permanent ponds, lakes, intermittent waters, ditches, irrigation canals, within underground aquifers, moist organic mats on forest floors and even in the cups of certain plants.
Ostracods have a range of conditions each can survive in, including salinity, temperature, and acidity. Knowing these tolerance levels allows scientists to make estimates of past climates from collections of ostracods shells found in wetland sediments.
Ostracod front view showing the animal inside the shell. Focus stack and dark-field microscopy. 100X.
Ostracods are primarily benthic animals and are rarely found in the plankton of water bodies. Most forms are free-living. The species may have a swimming, clinging, climbing or burrowing life style. Because swimming is energetically demanding, most ostracods can only swim short distances.
The greater majority of the ostracods are detritovores and herbivores. Their diet is restricted to algae and organic detritus. A few are known to be carnivorous. Some ostracod taxa are opportunistic predators and display foraging behaviour. For example, Australocypyris insularis may influence the structure of the aquatic microfaunal community in saline lakes. Other ostracod species have been observed to consume snails. Four species have been shown to consume amphibian eggs consuming up to 90% of the egg mass.
Ostracod, note the eye spot at the top. 100X DIC microscopy
Physical and chemical characteristics of the aquatic habitat are important factors in ostracod distribution and abundance. A high percentage of the ostracods are found only in bicarbonate rich water. Bicarbonate after being converted to carbonate is the prime constituent of the ostracod shell.
Ostracods are typically identified taxonomically from adult specimens. There are approximately 200 species identified from Australia.
Why include microfaunal community assessments in studies of environmental impacts and water quality?
Aquatic microfauna are an extremely important link in the aquatic food web, serving both as consumers- of algae, bacteria, other microorganisms, plus larval macro-invertebrates and fish fry: - and as prey for larger animals such as macro-invertebrates, small fish species and the juvenile stages of larger species, and many water birds.
Aquatic microfauna are an important part of the diet of birds. For example the diet of swans consists mainly of aquatic vegetation, however they are also insectivores as young swans (cygnets). In particular, cygnets need a protein rich diet, consuming small crustaceans and insects as their main source of food. Similarly ducks and geese also consume a wide variety of aquatic plant material, plus associated microcrustaceans.
Therefore the presence or absence of microcustacean species in a water body may provide us with useful ecological information. In addition the inclusion of aquatic microfaunal community analysis can help provide a more thorough understanding of water quality.
In some inland waters, the relative abundance of microfaunal species or types can be used as an indicator of water quality. Some species are limited by physicochemical variables, such as oxygen, temperature, pH or salinity. Microfauna are also affected by competition between species, predation by other species, and food availability.
The microfaunal species composition in a particular consistent water body usually remains somewhat stable over time, and the sudden appearance of new species or disappearance of existing species may indicate a change in water quality due to toxic substances, eutrophication, or imbalance between piscivorous and planktivorous fish; or as in the case of large or deep reservoirs, due to a flushing event or a turn–over event following thermal/chemical stratification.In temporary water bodies or those subject to wide fluctuation in water levels, the changes in temperature, nutrients, ionic content, pH and the underwater light regime influence on aquatic plants and algae, may also influence microfaunal species type and number of individuals per species that may be present in the water body at a given point in time.
Therefore a routine monitoring program involving the aquatic microfauna, utilizing proper scientific sampling techniques is recommended. There are different methods used to sample particular habitats and in sampling for Rotifera and Microcrustacea.
Continuous, long-term monitoring of microfauna community structure are useful in detecting patterns and changes in species composition, these in turn may be related to changes in water quality. A routine monitoring program also helps to separate the ordinary effects of seasonal changes upon the microfaunal community from changes caused by other factors.In short the aquatic microfauna are arguably the most important link in the aquatic food web. As a group they link all the components in the aquatic food web together.
However to-date the use of these animal groups in aquatic ecological studies in Australia has been overlooked for a variety of reasons. This may include the lack of knowledge and awareness of the importance of this group of animals in aquatic ecology by managers and decision makers. The lack of suitably trained personnel and resourced laboratories to inform the managers and decision makers. In addition to the lack of funding by both State and Federal Governments, and the prioritization of funding away from life sciences.
Managers of wetlands, waterways, reservoirs, water and sewerage treatment plants, need an understanding of the environmental baseline conditions and ecology of these waters that influence these animals.
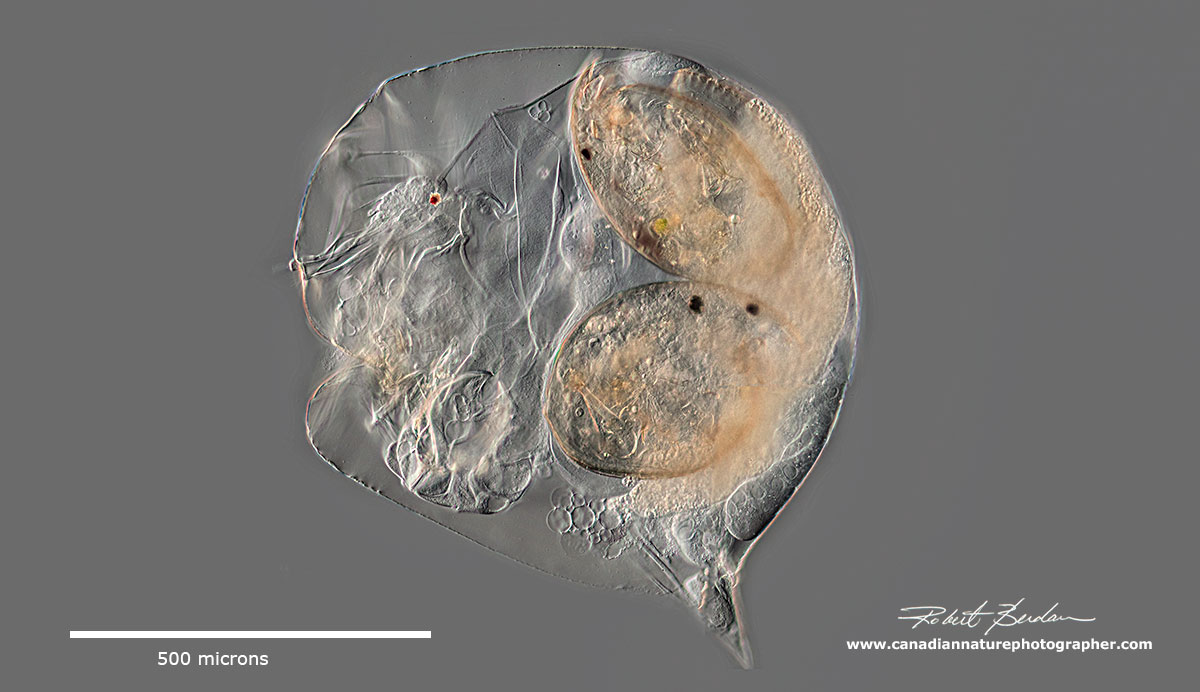
Photo of member of the family Chydoridae from Canada by DIC microscopy 100X.
If not monitored properly these waters (lakes, dams, reservoirs, wetlands, etc) have the danger of being nothing more than endpoints for drains. For example blue green algae events. The microfauna are a really fundamental link in the ecology of these systems and play a vital role in keeping algal blooms in check as this forms a major component of many species diet. It all comes back to environmental monitoring over the long term, then remedial action may take place to address the reported conditions.
The on-going ecological monitoring with the use of aquatic microfauna of both temporary and permanent waterways, water impoundments, reservoirs of all types, and stormwater holding ponds; will provide information relating to their health, plus also to inform managers of changes in previously recorded baseline conditions of these environments. In short this is something that will aid in obtaining environmental approvals from regulators, allow the selling of projects to stakeholders, give them a positive spin/media release etc.
This has implications for environmental conditions, waterway assessment and management, mining industry (mine lakes), ecological services, stormwater management, surface and ground water assessments etc.
To put it briefly, the aquatic microfauna are arguably the most important link in the aquatic food web. As a group they link all the components in the food web, living and non-living together.
Authors Biography & Contact Information
 Dr. Robert Walsh is an aquatic micro-invertebrate ecologist/taxonomist in Australia. He operates a small environmental consulting firm with a fast turn around and specializes in identification of zoo plankton and aquatic micro-fauna. He is also involved in education about the biodiversity of micro-plankton and its importance.
Dr. Robert Walsh is an aquatic micro-invertebrate ecologist/taxonomist in Australia. He operates a small environmental consulting firm with a fast turn around and specializes in identification of zoo plankton and aquatic micro-fauna. He is also involved in education about the biodiversity of micro-plankton and its importance.
Address: 55 Vaughan Chase, Wyndham Vale, VIC 3024, Australia
Email at: rwalsh@australianwaterlife.com.au
Web site: www.australianwaterlife.com.au
Phone International: +61-3-8742-6397
Mobile: 0427-431-041
Local: (03) 8742-6397