
Crystals Photographed with Polarization Microscopy: Water, Beer, Caffeine, Vitamins, Amino Acids and Human Tears
by Dr. Robert Berdan
March 17, 2019

Vitamin C crystals viewed by Polarized light Microscopy 100X
Introduction
Crystals viewed and photographed through a polarized light microscope can display the most intense and beautiful colours I have ever seen in nature. One can view minerals and crystals formed by both plants and animals. Other substances like vitamins, drugs, house hold cleaners, beer, wine, alcoholic drinks, sport drinks, starch grains, hair, wool fibers, and even tap water can produce beautiful colours. Polarized light microscopy has played an important in role in the identification of minerals, fibres and other substances in forensic science and biological research and several photographers have used polarized light microscopy to produce art. Polarized light microscopy is a clear example where both science and art come together - see my article entitled Marriage of Science and Art through microscopy.

Above Vitamin C crystals viewed by Polarized light microscopy 100X
Colours in large crystals (e.g. mica) can be seen between polarizers without a microscope, medium sized crystals can be viewed using a stereo microscope and small crystals down to the size of about 1 micron (0.001 mm) can be studied with a light microscope. It is easy to convert a basic light microscope into a polarizing microscope (watch video by the Microbe hunter Oliver Kim on YouTube) and also a stereo microscope (see below). In this article I will share the techniques I use to create crystals for photomicrography. I explain how to make crystals on microscope slides and show examples in a previous article on this site. I also will provide information on different methods of crystallization used and describe the solvents I use. My hope is that you might become inspired to try viewing and photographing crystals with a polarized light microscope.

Vitamin C forms a wide variety of crystal shapes and colours - these resemble flowers 100X
To examine micro-crystals a polarizing microscope is required, but any bright field microscope can be converted into a polarizing microscope by adding a polarizer over the light source and placing another polarizer called the analyzer in the eyepiece. A even simpler procedure is to put a polarizer below the microscope slide and one above the slide - watch video by Oliver Kim on YouTube on how to do this using polarizers cut from a pair of cheap 3D glasses. The polarizers must be linear not the circular type.
Research quality polarizing microscopes used by scientists and technicians have additional accessories such as a rotating stage and retardation plates, a quartz wedge and possibly other compensators (see Nikon Microscopy U). The colours observed provide important information about the specimen including how the molecules are packed and if the thickness of the specimen is known, one can also determine the identify of the substance using a Michel-Lévy Color Chart (see below) which identifies the substances based on their birefringence (difference between two refractive indices). If you would like to know more information about crystal birefringence please see many of the articles available on the web - here is a short review of Polarized light Microscopy by Robert Bagnall 2012 - PDF to get you started.
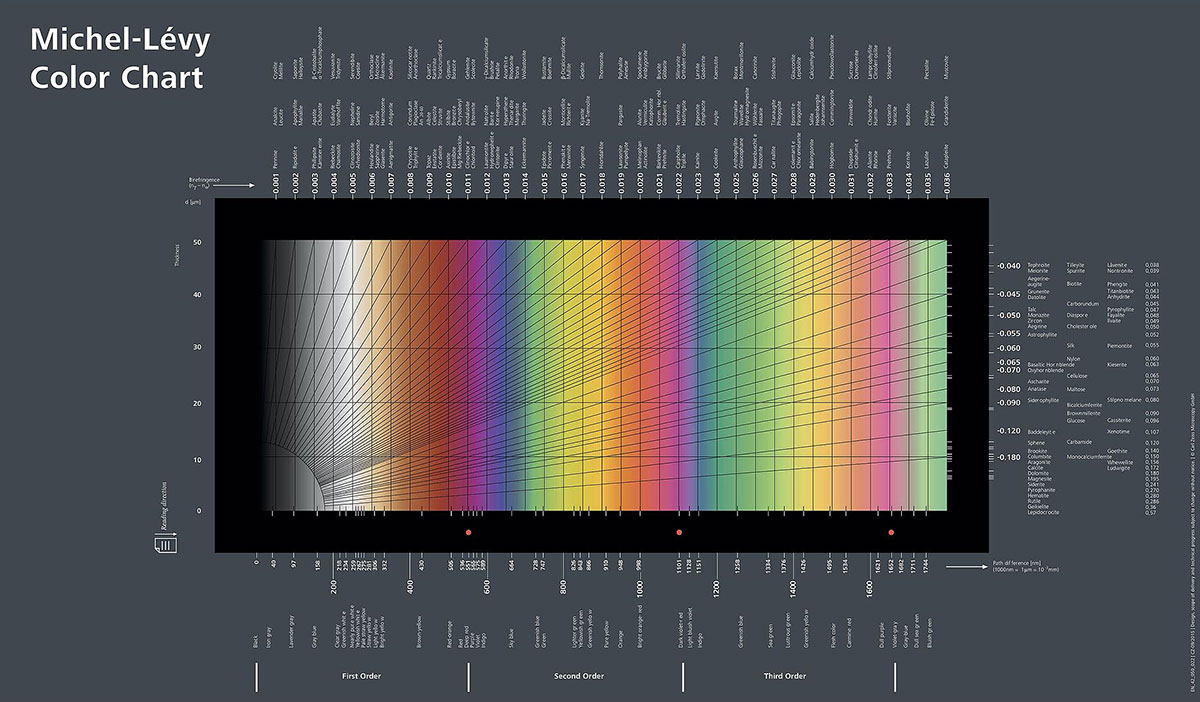
Michel-Lévy interference colour chart provided by Carl Zeiss Microscopy - via Wikipedia.
On the x-axis of the chart above is the thickness of the specimen (in microns), the colours are what you see when viewing birefringent specimens through polarized light. The colours on the y axis become more faint and subdued towards the right and they are referred to as increasing orders. As the specimen gets thicker the colours also become more subdued - see crystal photos below. The colours are due to the interference of light as they leave anisotropic (birefringent) substances (substances that have 2 or 3 refractive indices). To learn more about how to use this chart see Polarized Light microscopy on Wikipedia and some of the references below.

On the left is a Motic SMZ161 stereo microscope polarizing Kit (cost about $130). You place this on your stereo microscope stage. You need a light source below it, LED lights for microscopes work well. Place your microscope slide on top of the bottom polarizing filter, rotate the top polarizing filter while viewing your specimen. M. Reese (2008) shows how to convert and use a stereo microscope to view crystals in polarized light if you like to create your own devices. On the right is Unitron Polarizing microscope from the 1960's, it has a Bertrand lens, built in retardation wave plate and a rotating stage. I purchased this older polarizing microscope for a reasonable price. The pictures in this article, however were taken using my Zeiss Axioscope research microscope and a Nikon D500 digital camera and Digicam control software which is free and the camera RAW files were processed in Adobe Photoshop.
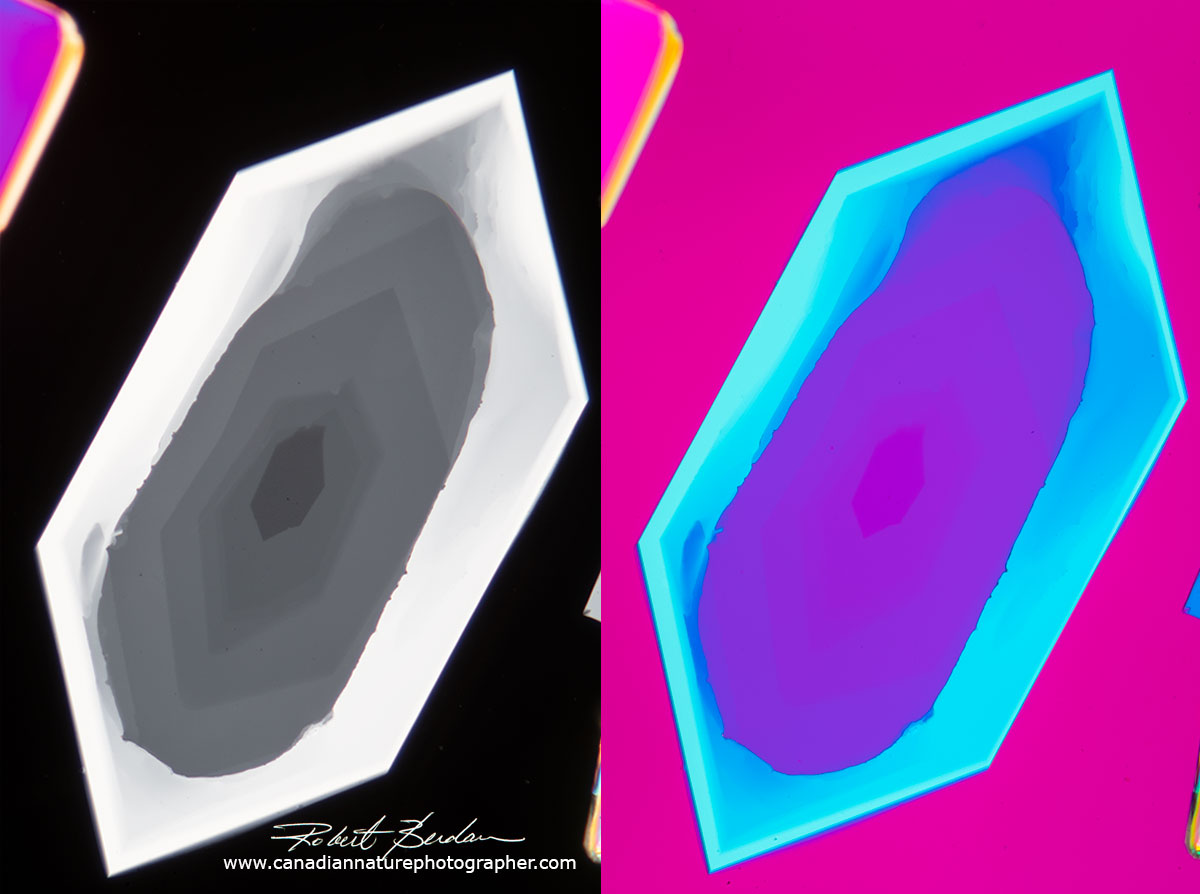
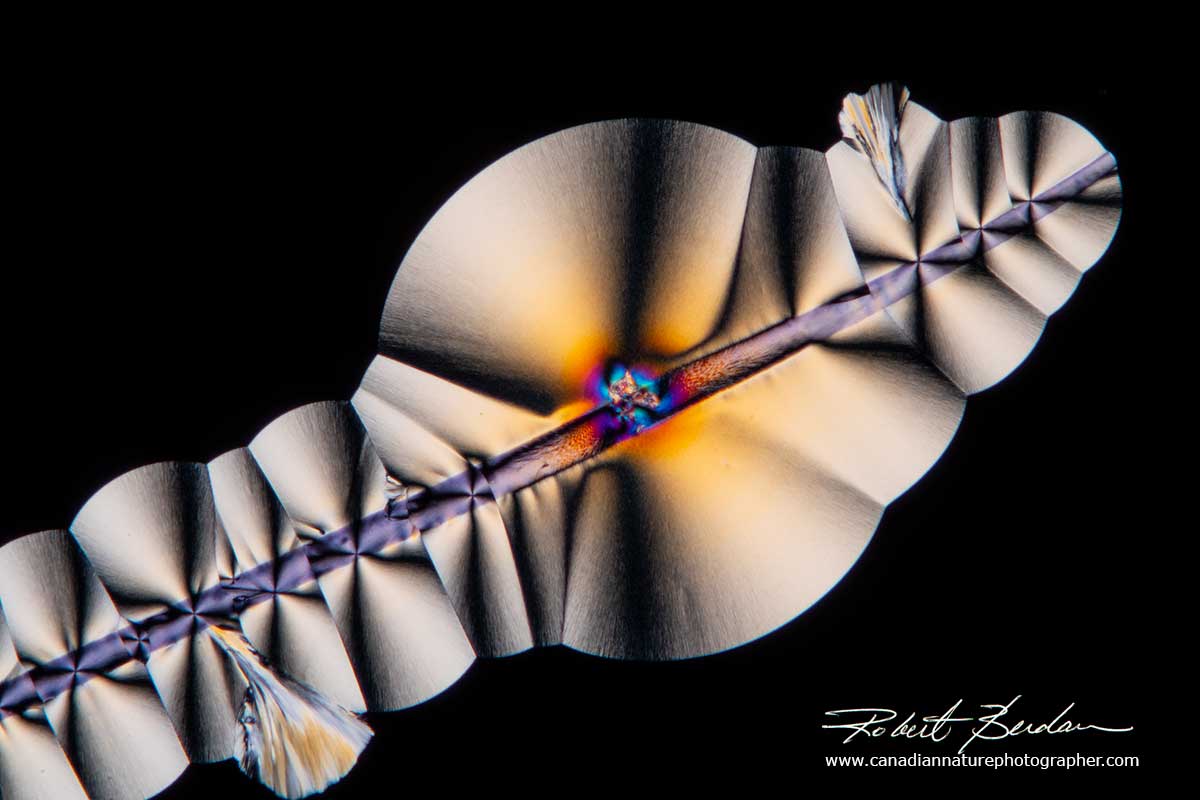
Sulfamic acid crystal on left viewed with polarized light and on the right viewed with polarized light and a full wave retardation filter - 100X. Retardation plates increase how much the light exiting the crystal is out of phase causing interference and different colours. They are some times called a red plate because of the background colour produced (which is more pink or magenta then red). Thin sheets of mica of known thickness (100 microns) are sometimes used as a full wave retardation plate and a quarter wave plate was 25 microns thick (micron is 0.001 mm - Knight Optical). You can even make your own retardation plates with mica or scotch brand transparent tape (not the tape you can write on).

Edge of a crystal of Vitamin C in polarized light showing interference colours near the edge due to differences in the thickness - compare colours with the Michel-Lévy interference colour chart above. The crystal becomes thicker toward the bottom left of the picture and the colours are more subdued - i.e. higher order interference colours.
Frozen Water
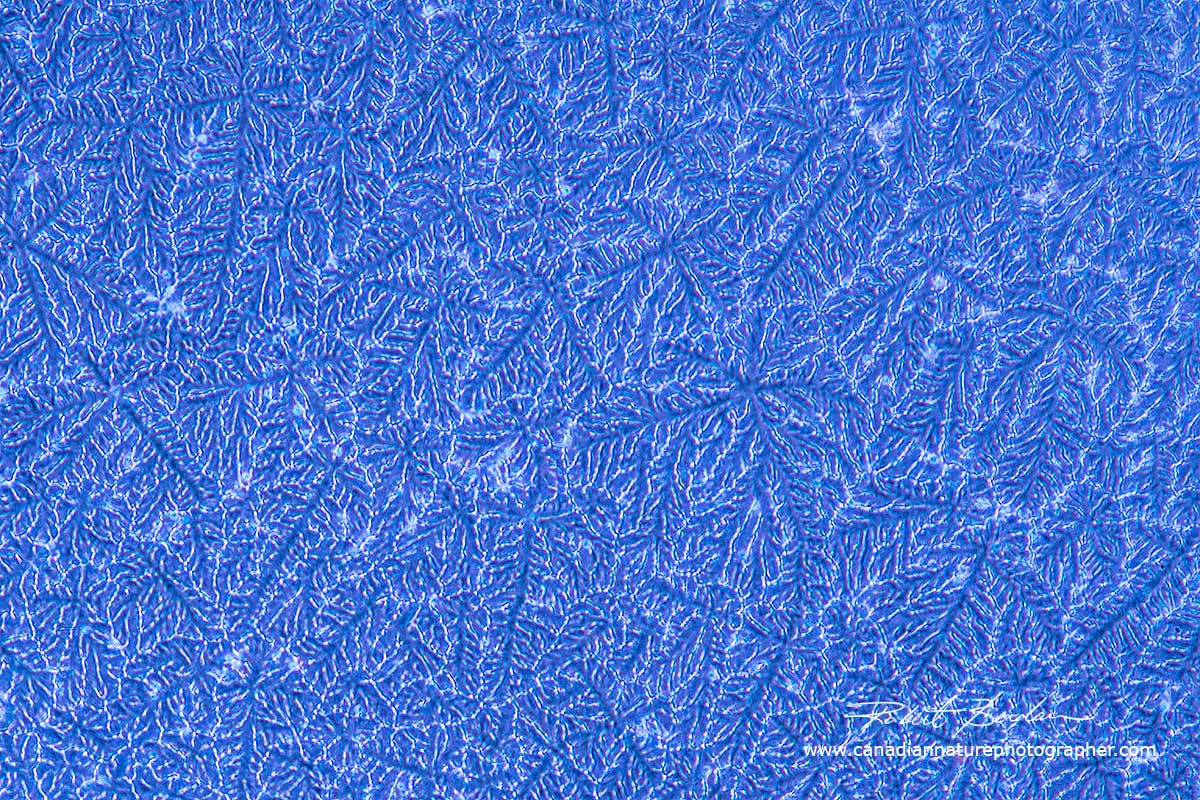
You don't need a microscope to view or photograph water crystals if you live in the higher latitudes just look out of a window on a cold winter day and use your camera and a normal lens or macro lens to capture their beauty. Depending on the humidity in the room the water will form a wide variety of fern-like crystals - see below.

Ice crystals on my kitchen window photographed in February with a 60 mm Macro lens.
If you examine frozen water through a microscope using polarizing filters and full wave retardation plate you will see colours that are due to the birefringence of ice - its ability to split polarized light into two components that interfere when the rays pass through the ice and polarizing filters. The colours also depend on the thickness of the ice. Ice crystals can be made by putting a drop of water on a microscope slide and putting the slide in a freezer for an hour. The challenge in photographing these small samples of ice is that they will melt quickly especially when placed under the microscope illumination - sometimes they melt within seconds. I generally get about 15-30 seconds to take photos before most of the droplet has melted.

Frozen tap water with air bubbles, polarized light microscopy including a full wave retardation plate. The water drop was allowed to freeze on the slide at -20 °C with no coverslip overtop of the water.
Frozen deionized water with a coverslip over top and photographed with 5X objective in polarizing light microscope. Deionized water seems to have fewer air bubbles. The crystals do not appear sharp in this picture because of condensation on the overlying coverslip. Some researchers use temperature controlled stages on their microscopes to keep the water frozen, but this kind of equipment is expensive (e.g. Microptik). At least one other scientist photographs frozen ice with polarized light and sells his images as art - Dr. Peter Wasilewiski at Frizion.com.

Snowflake photographed with the Canon MP65-E macro lens. This snowflake was collected in my backyard on a glass plate.
Snowflakes can be photographed with a microscope or macro lens - see article by Don Komarechka on this site. I will post an article in the future showing snowflakes photographed in polarized light and DIC.
Photomicrography of Crystals in Human Tear Drops
Dutch artist Maurice Mikkers has photographed human tears using Darkfield microscopy in black and white and suggests that they may be as unique as snowflakes. Research indicates that we produce three main types of tears: 1) basal tears that keep our eyes moist 2) reflex tears in response to irritation like vapours from a freshly cut onion and 3) emotional tears linked to sadness. Researchers have analyzed the contents of tears in terms of salts, proteins and sugars (Lam et. al. 2014, Zhou et. al. 2006). I have just begun to look at human tear drops using my microscopes which allows me to capture images of the crystals in colour. Crystals that I see in the drops are similar to those formed by crystals of salt (NaCl - sodium chloride) and salt appears to be a major component in tears. Below are a couple of photomicrographs of tears provided by my wife and you can see crystals inside the drops at higher magnification.
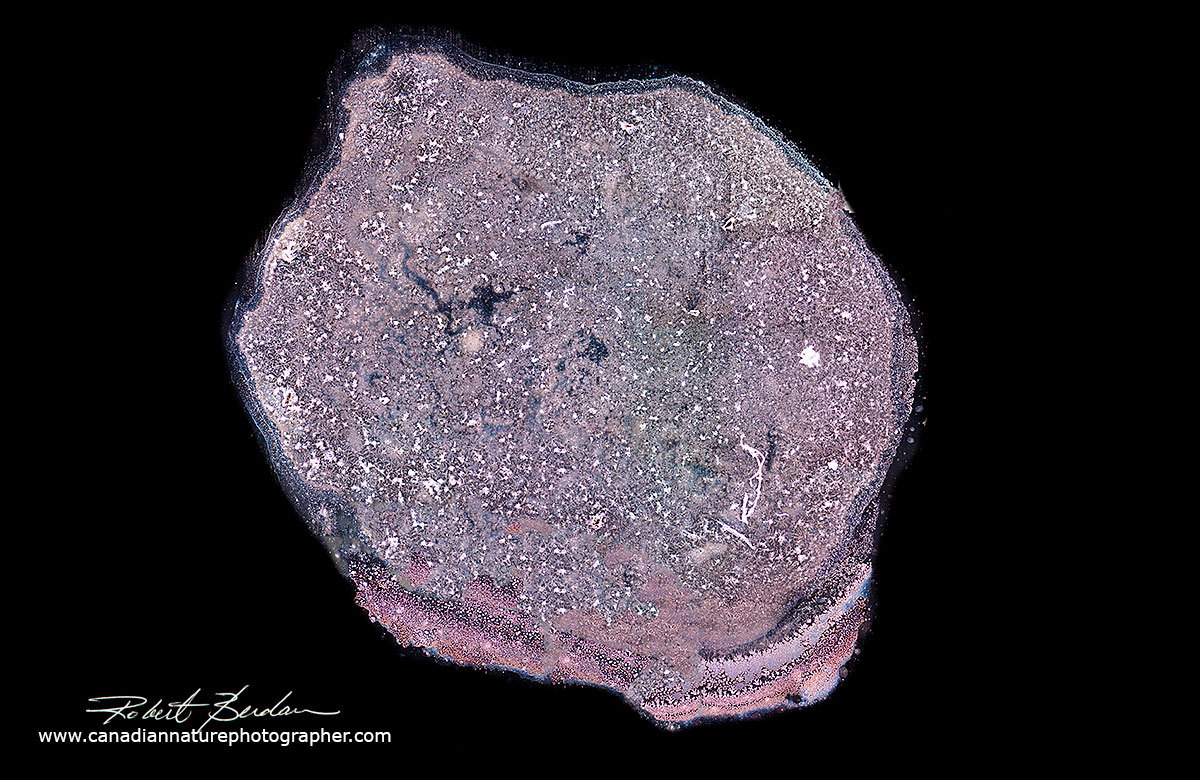
Above tear drop from my wife photographed with Darkfield microscopy about 10X.

Edge of a tear drop showing crystals inside the drop - viewed 40X using DIC microscopy
Crystals in a tear drop dried on a microscope slide - 100X DIC microscopy.
Vitamin C Crystals in Polarized Light
Vitamin C, also known as ascorbic acid, is economical and anyone can purchase the pure powder in pharmacies and health food stores. Vitamin C was essential to prevent scurvy in sailors that did not have access to citrus fruits or fresh meat in the past. The British navy used to store lemon and lime juice in barrels painted with green in their sailing ships. The juice was rationed to the sailors (along with Rum & water called Grog) to prevent scurvy and it is why the sailors and the British came to be called limeys. Indigenous people in North America avoided scurvy by eating fresh meat and the Iroquois used a decoction from the bark and leaves from an evergreen tree (Annedda or more likely Abies balsamea) D. J. Durzan 2009 J Ethnobiol Ethnomed. 2009; 5:5 - PDF. In 1937 the Nobel Prizes for chemistry and medicine were awarded to Haworth and Szent-Györgyi, respectively for their work on the purification of Vitamin C.
Vitamin C produces the most beautiful crystals I have seen by polarizing microscopy. The vitamin also forms a wide variety of different crystals depending on how fast it cools, crystal seeds, hair, dust, and proximity to the edge of the coverslip. I start by making a saturated solution of vitamin C powder in water and isopropyl alcohol (1:1) and applying the solution to a microscope slide. Crystallizes can appear in a few minutes when air dried and even faster by heating the slide on a hot plate. According to A. Shalamis and A. Eliassi (2008) J. Chem Eng. Data - PDF - vitamin C solubuility is greatest in the following solvents: 1) Water 2) Methanol 3 ) Ethanol, 4) Propan-2-ol (isopropyl alcohol), 5) Acetone, 6) Acetonitrile, 7) Ethyl Acetate and 8) Tetrahydrofuran - see Table 1 in their publication. You can also melt Vitamin C powder at 190 °C on the slide to make crystals directly using the melt method. When making crystals from a solution I get best results when I allow the slides to air dry slowly for a few hours.
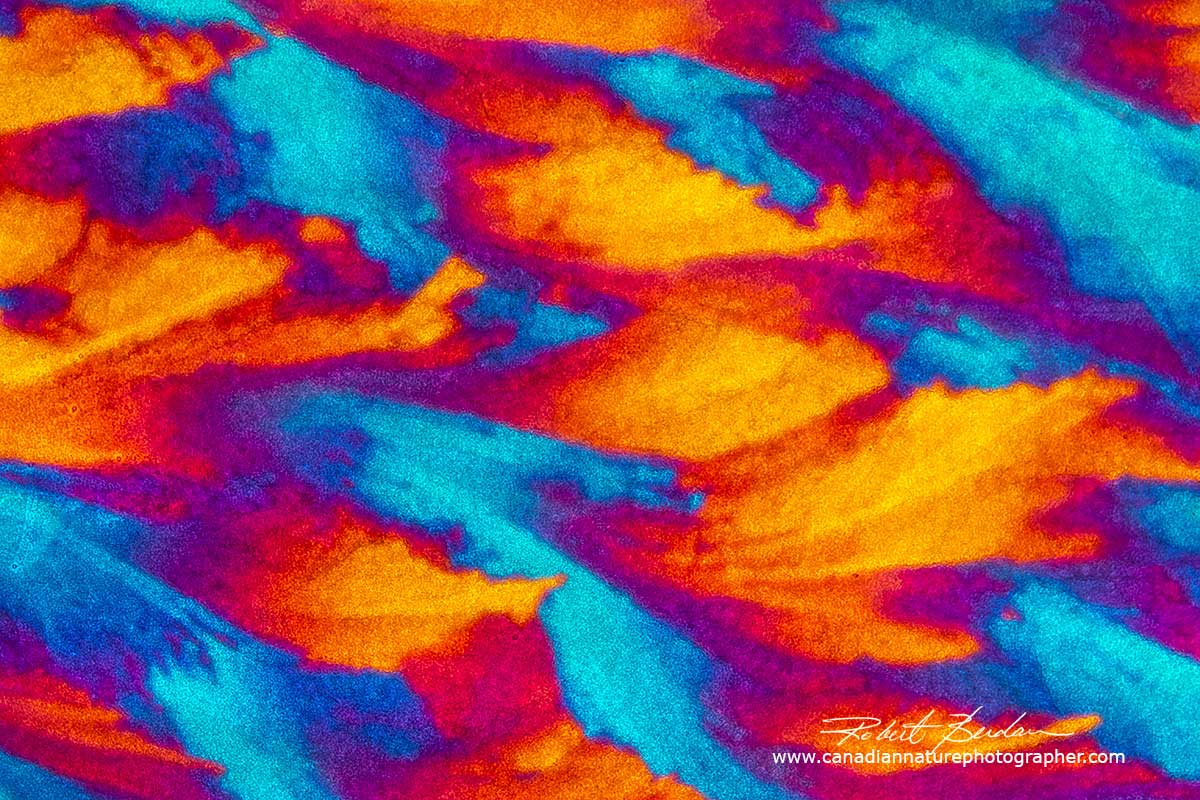
Below are some photomicrographs of Vitamin C with polarized light microscopy - it is now my favourite chemical to make crystals. The crystals look best using low power microscope objectives like 2.5, 4 and 10X. You will also note that the spherulites (circles with rings) are common, but vitamin C also forms a wide variety of other crystal shapes and the crystals can appear metallic silver and gold.

Vitamin C by Polarized light microscopy and a retardation plate 100X.

Several Vitamin C crystals by Polarized light microscopy 100X

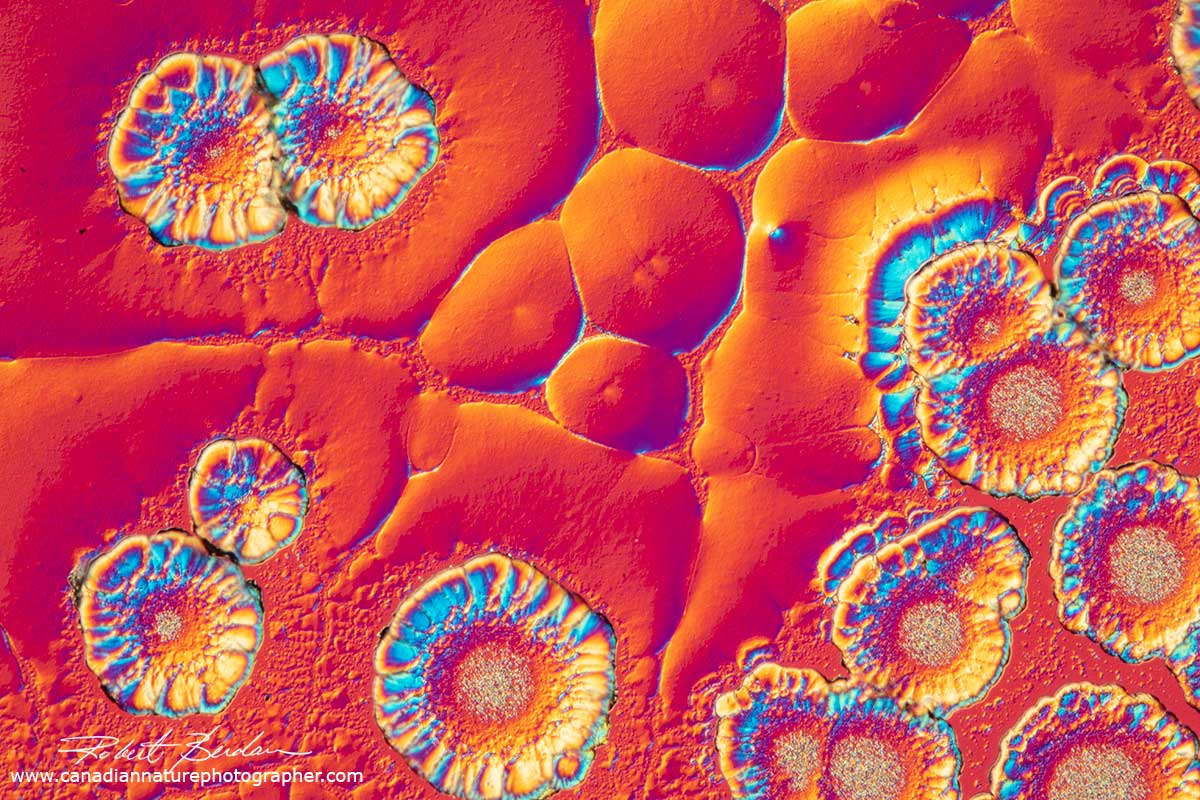
Vitamin C crystals that appear flower-like by polarized light microscopy 100X.

Vitamin C crystals with 3 circular crystals (spherulites) with dark crosses inside.

Single vitamin C crystal with a circular shape (Spherulite), complex pattern inside and Maltese cross - Polarized light 100X

Two spherulites of Vitamin C attached like budding cells, 100X.
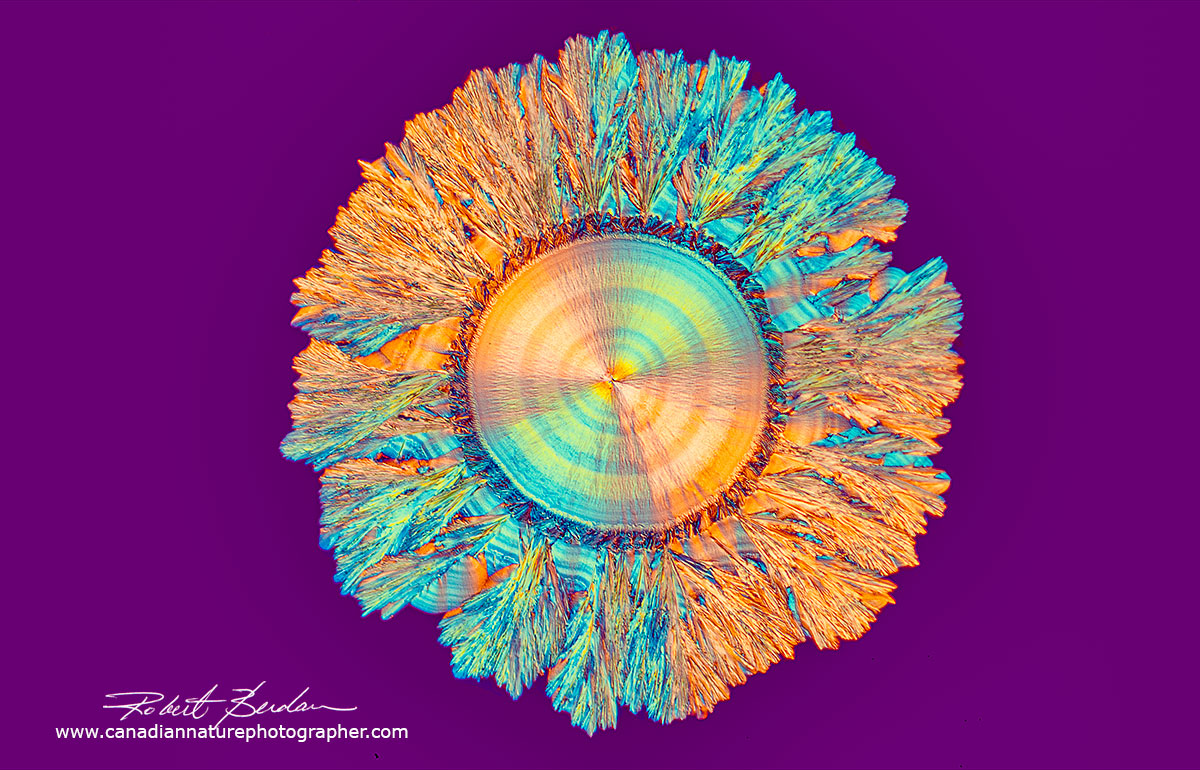
Sun shaped spherulite of Vitamin C 100X. DIC microscopy.
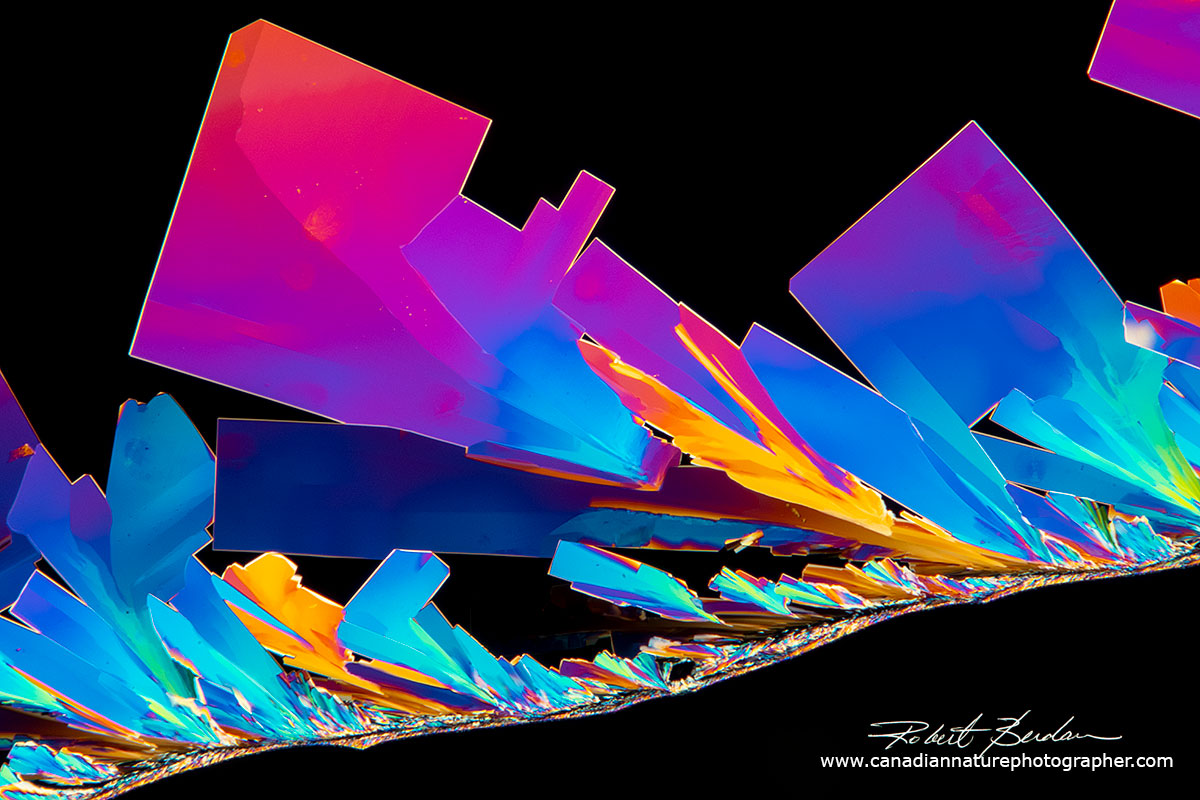
Vitamin C crystals forming along a hair - Polarized light microscopy.

Large panorama made up of a dozen photos stitched together - Vitamin C Polarized Light. By stitching together many photos in Photoshop it is possible to make very large prints from the images.
Another kind of microscope that can be used to view crystals is one with Differential interference - or DIC. These microscopes are not common because they are expensive and adding this feature to an existing microscope can cost over $10,000. The advantage of this technique is that this microscope can add colour or 3D-like relief even to isotropic crystals which generally do not produce colour under a polarizing microscope e.g. salt.

Table salt (sodium chloride or NaCl) is isotropic and not birefringent and does not produce interference colours when viewed in polarized light (below) or by DIC (above). 100X. Salt can form other crystal shapes called Hopper crystals.

Table salt crystal viewed by polarized light microscopy - focus stack 400X.
The DIC microscope can also be used as a polarizing microscope as it uses polarized light, but it has special prisms that split polarized light, but these prisms can be removed from the light path. For more information on how a DIC microscope works see the reference above.
Methods for producing Crystals for Polarizing Microscopy
1. Melts - put some of the powder on a microscope slide, add a cover slip and place the slide on a hot plate or flame from a Bunsen burner. This works very well with substances having a low melting point (e.g. ascorbic acid, citric acid and caffeine) and has the advantage that you can melt the substance several times to get different crystals. Some folks smear or move the coverslip after melting the powder so this spreads the molten material and it can result in thinner or flatter crystals. Some substances don't melt cleanly but burn and turn brown.
2. Create a supersaturated solution of the powder in a solvent, water, isopropyl alcohol, acetone or other solvent or mixture then place several drops of the solution on a microscope slide and let it air dry, or you can heat the slide on a hot plate until it evaporates. I usually put some sample on a slide in a drop by itself and I also put some solution under a coverslip so it dries more slowly. Often I get crystals forming around the coverslip edge, but not under the coverslip. Sometimes you need to let the solution crystallize overnight or even longer.
3. Make a solution and freeze (I use a - 20°C freezer or I can put samples outside in winter). This technique works with most solutions including beer, soft drinks etc. The main disadvantage is that the solution melts in seconds under a microscope without a temperature controlled stage and the images generally don't contain fine detail (see below). I have been thinking of trying to freeze the solutions with dry ice but haven't had a chance to do this yet.
4. Concentrate the solution (to achieve a supersaturated solution). I usually do this by boiling solution and then allow the solution to dry on a slide in air or under a coverslip. Some researchers also add different salts or precipitants (e.g. Ammonium sulphate or Polyethylene glycol) which reduces the solubility of the substance and in turn promotes nucleation and crystal growth.
5. Add a seed. A seed is a substance that promotes nucleation of crystals. The best seeds are usually microcrystals of the substance or in the case of caffeine one can add some powdered caffeine to the solution to promote caffeine in the solution to crystallize. Some scientists use grains of sand, glass chips, hair and even scratch glass with a diamond pen to stimulate crystallization. Scientists have also grown crystals in zero gravity to see if they can grow bigger crystals used for determining the molecular structure of proteins by X-ray diffraction.
Vitamin C crystals growing on an small hair which acts as a seed - Polarized light microscopy 100X.
6. Vapour diffusion method is widely used to produce protein crystals. A drop of solution usually with a seed is placed on an inverted coverslip in chamber with saturated protein solution in the bottom. In my brief experience using this method with beer so far I have only been able to grow mold in the drop. It seems essential to keep the solutions sterile to prevent the growth of unwanted substances. I plan to keep trying this technique until I am successful.
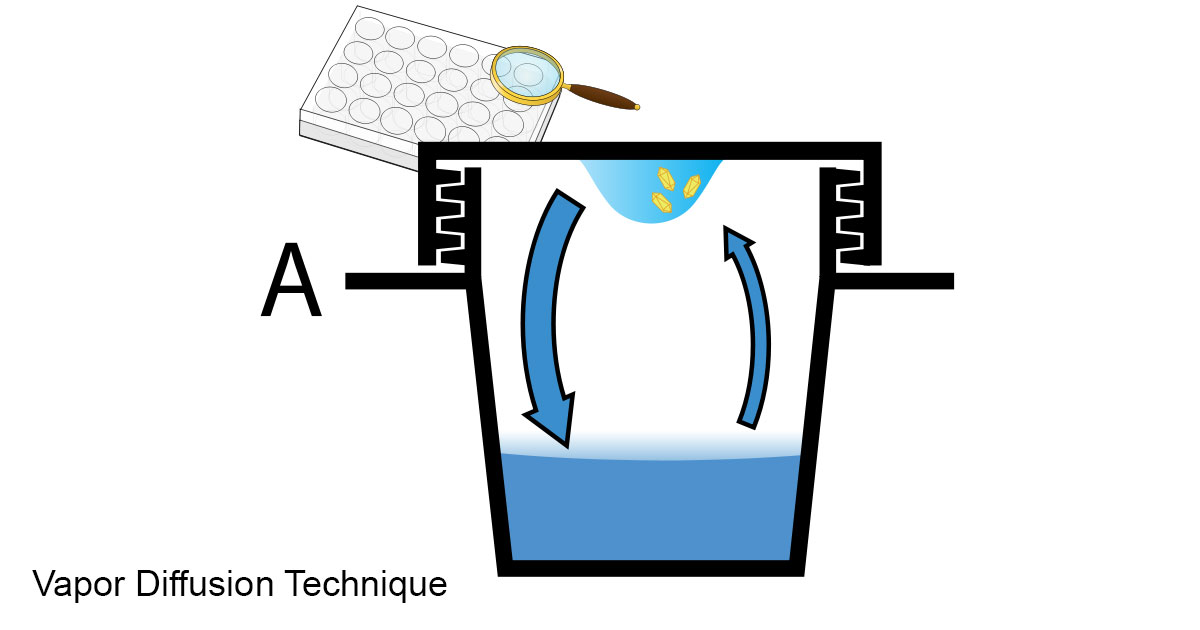
Diagram from Wikipedia - this method is often used to grow protein crystals which are used for X-ray diffraction studies to determine the molecular structure of the protein.
Some crystals are easy to produce in a few seconds e.g. Epsom Salts (Magnesium sulphate) and others like growing crystals from beer can take weeks. It is important to be aware of that many compounds form more than one type of crystal e.g. NaCl (table salt) that can take on a wide variety of crystal shapes (J. Desarnaud et. al. 2018). Solutions containing other salts or components can influence (inhibit or promote) crystal growth and in turn affect the appearance of crystals.
The simplest method to make crystals for polarized light microscopy is to make a concentrated solution in water - heating the water or using a vortex mixer promotes solubilization. You can use tap water or deionized water. I use both as sometimes impurities in tap water seem to promote crystallization. You can buy deionized water in jugs at your local drug or food store. Allow the sample to air-dry or heat a drop of the solution on a microscope slide with a hot plate and then view the crystals with a microscope. Another simple method is to melt the crystals if it has a low melting point (i.e. below about 250 °C). I use both methods because each technique often produces different looking crystals. For example caffeine in water results in needle like crystals, whereas when melted it forms flat map-like crystals in polarized light.
The use of correct thickness coverglass is recommended (i.e. No 1.5 thickness is ideal 0.17 mm +\- 0.005 mm thickness - I buy mine from Zeiss) but in practice I find that for objectives 10X and below using a coverglass is not essential. They are more important when using 20, 40X or higher power objectives. I also use focus-stacking to photograph the crystlas if they are thicker then the depth of field.

Needle-like caffeine crystals from Rockstar energy drink 100X.
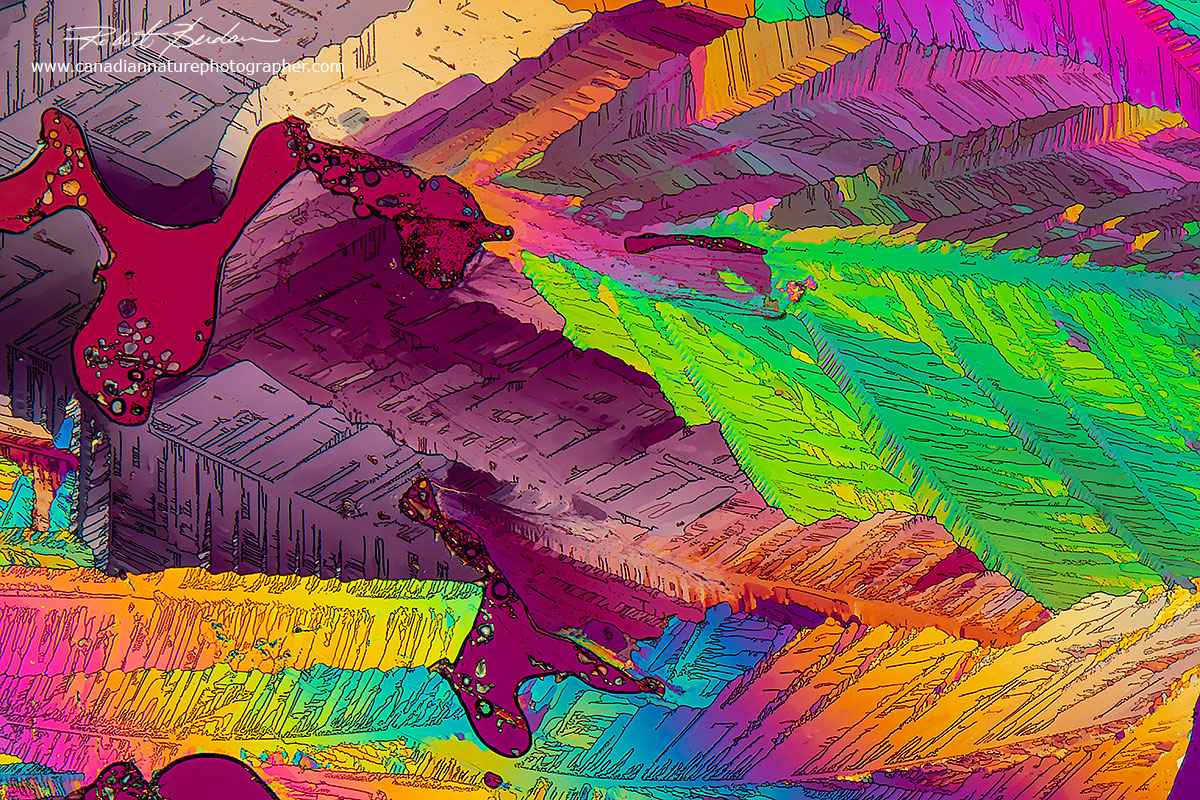
Flat map-like crystals of caffeine produced with the melt method 100X

Above polarizing photomicrographs of citric acid made using the melt procedure. Citric acid is a weak organic acid found in fruits. It is highly soluble in water and has a melting point of 156 °C

Citric acid crystals in Polarized light microscopy - melt method 100X
Sourcing Chemicals
You will likely have a wide variety of chemicals in your home e.g. bleach, mothballs, cleaners, various medicines, Epsom salt etc. Start trying to make crystals with these chemicals. You may want to wear rubber gloves, protective clothing (I have a lab coat) and a pair of protective glasses is recommended - sometimes the coverslip explodes off the slide when heated. My experience with household medicines is that many did not produce good crystals because often other components are added to protect your stomach or inhibit crystallization. Try to get pure chemicals if you can. Pure chemicals are available online, and from some Medicine shoppes, health food stores, drug stores, hardware stores and online science suppliers e.g. Home Science tools. A mortar and pestle can be used to crush pills or anything you want to extract fluids or salts from and is available online e.g. Amazon for about $20. The first two chemicals I would recommend are Vitamin C and Citric acid - try both melts and air drying from saturated solutions. There are hundreds of other compounds you can test. It is a good idea to read up on the chemicals before you work with them to learn about their solubility, melting point and whether or not they might be toxic or produce toxic gases when heated. Material Safety Data sheets (MSDS) are readily available online and describe the potential hazards chemicals might have. You only need very small amounts of the chemicals. Young children should be supervised when experimenting with chemicals.

Citric Acid crystals viewed by polarizing light microscopy using the melt method.

Panoramic photo of Vitamin C composite image made up of 27 different images and is over 5 x 7 feet. Polarized light microscopy. By making panoramas it is possible to make very large prints.

Panorama of Vitamin C crystals in the style of David Hockney - to learn how to make these type of images see my article on how to simulate "David Hockney" style photographs using Photoshop

Vitamin C crystals in polarized light 50X
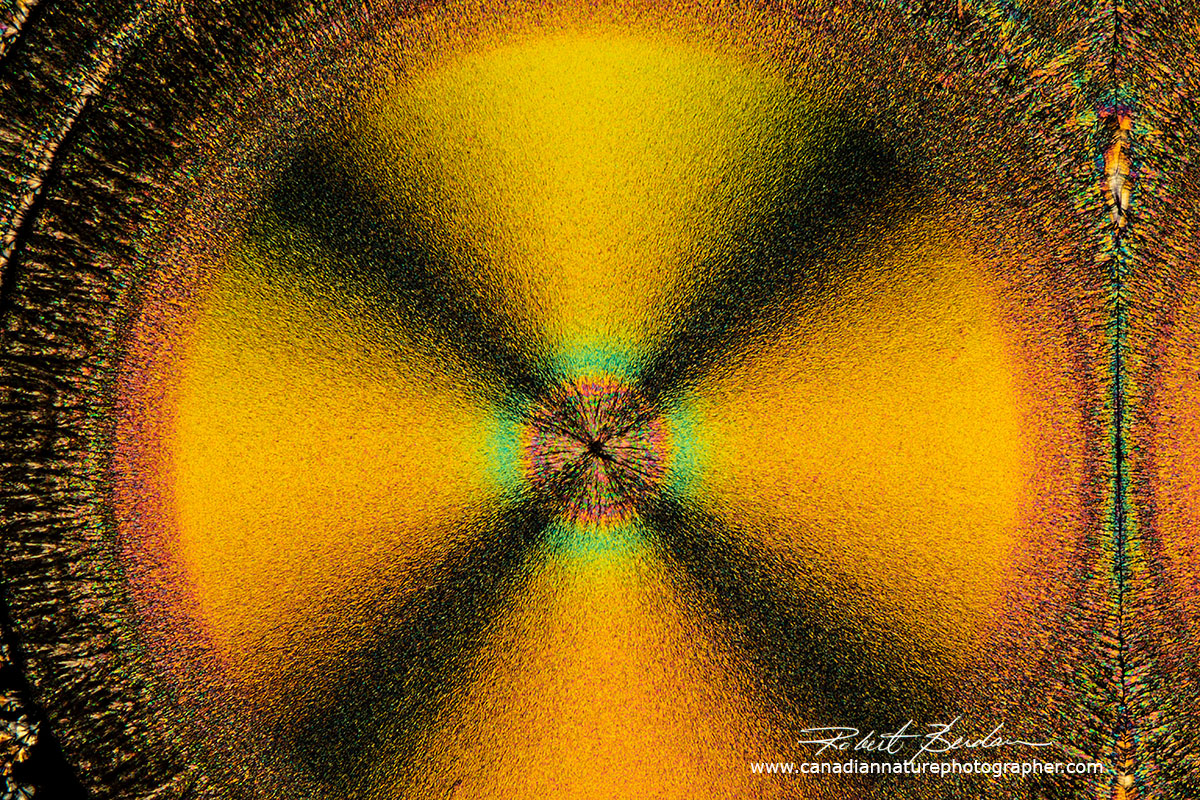
Vitamin C crystal showing the "Maltese cross" which represents the regions where the crossed polarized light is visible i.e. the dark background blocked by crossed polarizers. If the direction of light coming through the crystal corresponds with that of one of the crossed polarizers then little light is transmitted and appears black. The round crystal is called a spherulite. Their formation is associated with melt crystallization and their size depends on the cooling rate. When the crystals are cooling in the absence of a thermal gradient growth occurs radially in all directions resulting in spherical aggregates called spherulites. The slower the cooling the larger the spherulites. Spherulites are composed of highly ordered lamellae and the alignment of the polymer molecules within the lamellae results in birefringence including a Maltese cross.
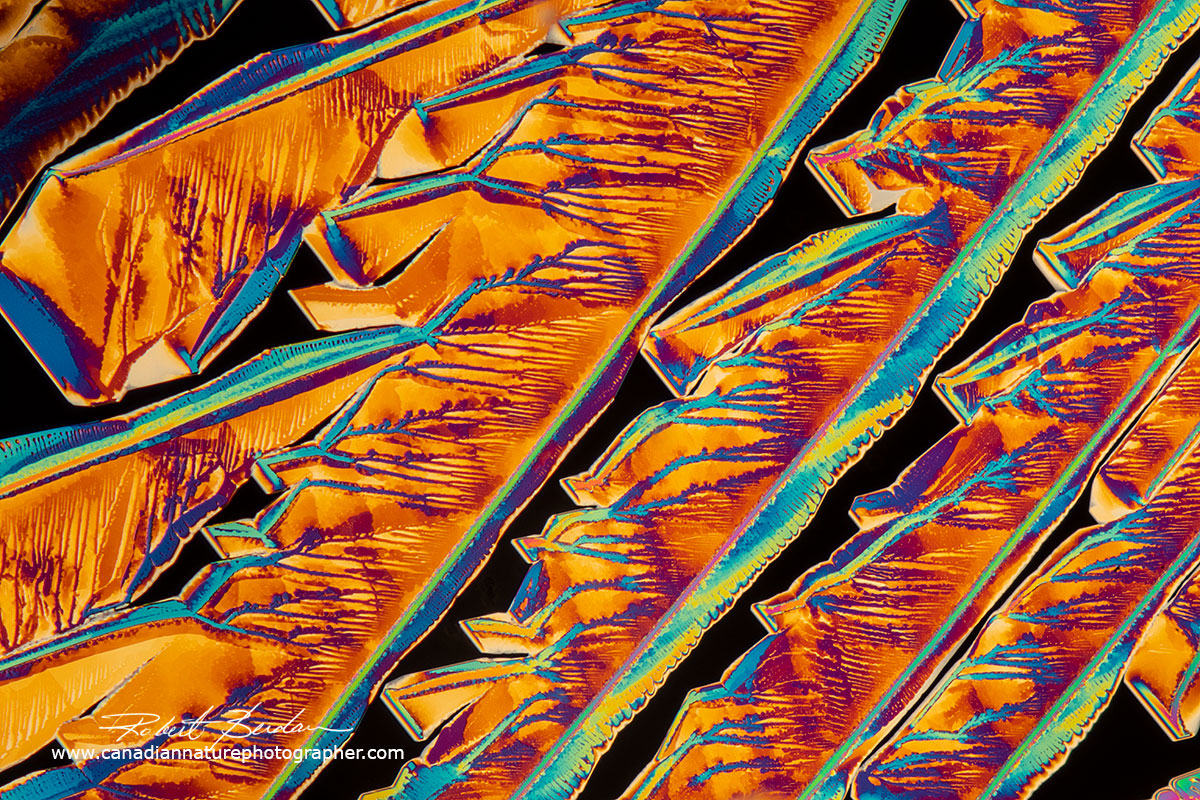
Zinc Acetate crystals produced by heating a saturated solution in water on a microscope slide - Polarized light microscopy 100X
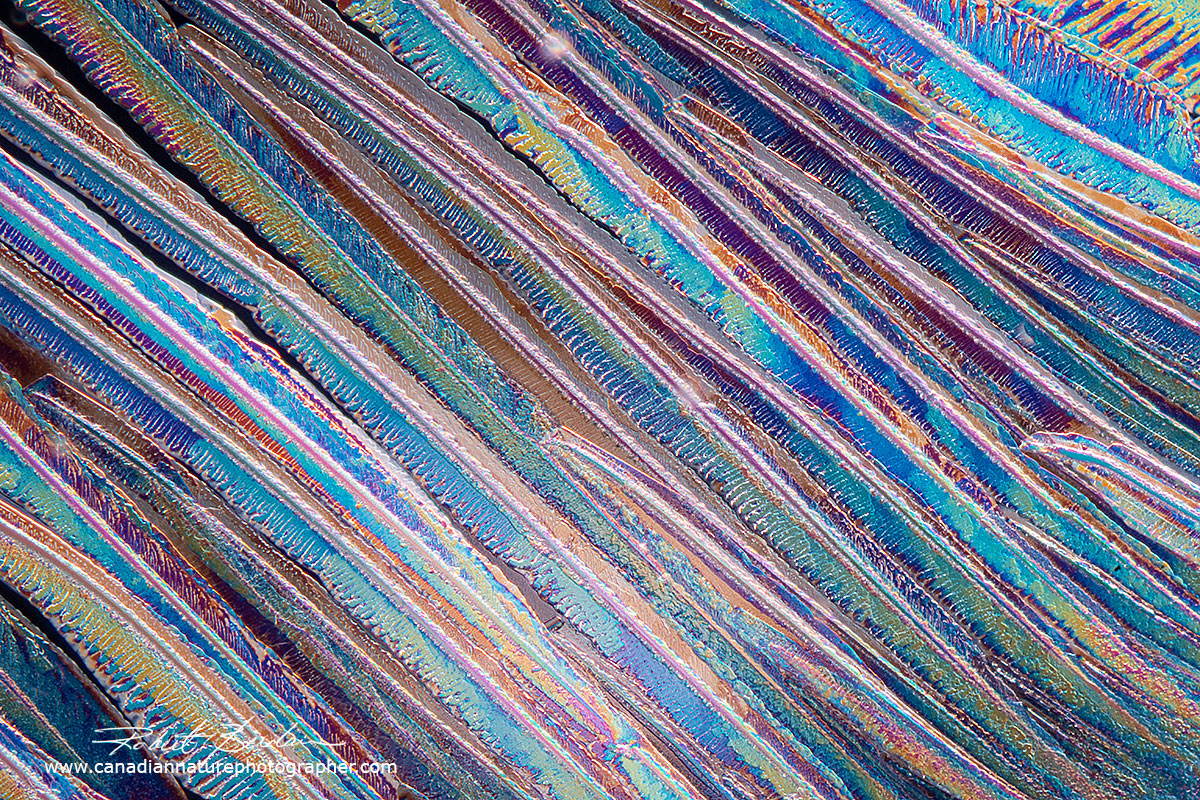
Zinc Acetate crystals dried from a solution. Zinc acetate is used in lozenges for treating the common cold and is used as a topical anti-itch ointment.
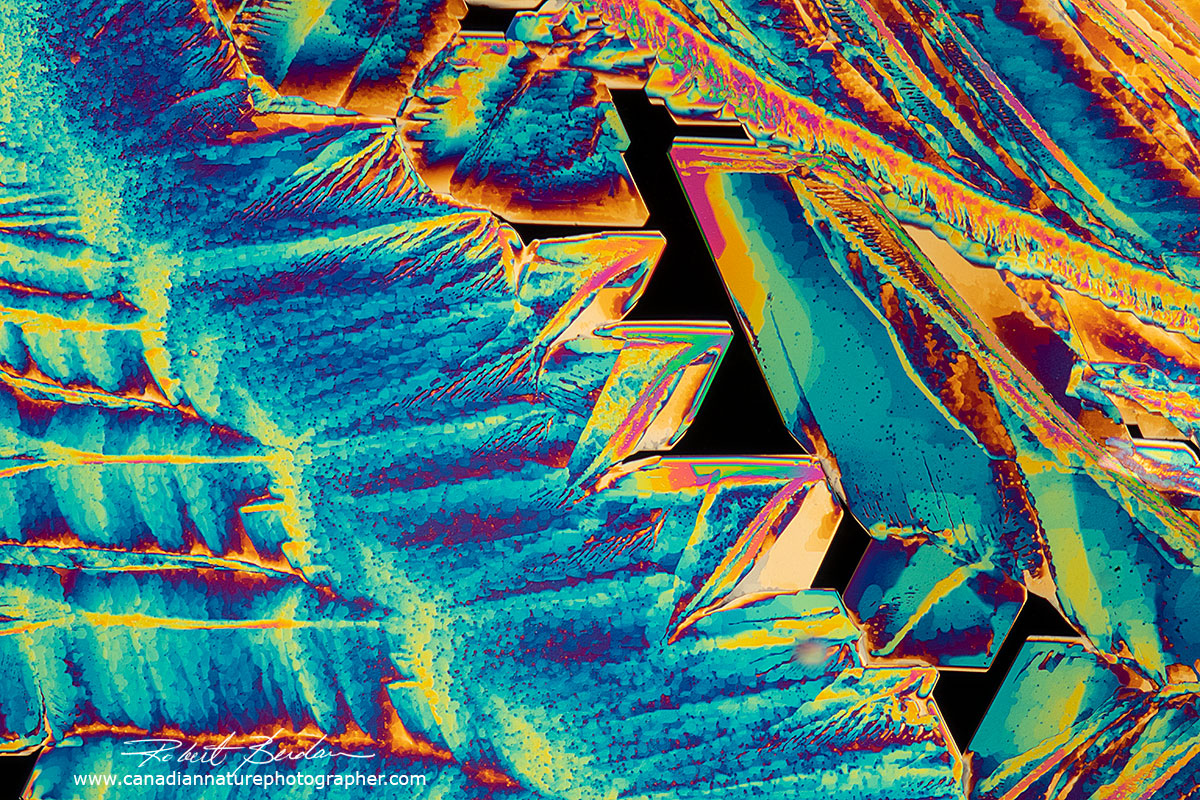
Above polarized light micrographs of crystals of Zinc Acetate

Urea crystal produced by melt method - polarizing microscopy. Urea serves an important role in the metabolism of nitrogen-containing compounds by animals and is the main nitrogen-containing substance in the urine of mammals.
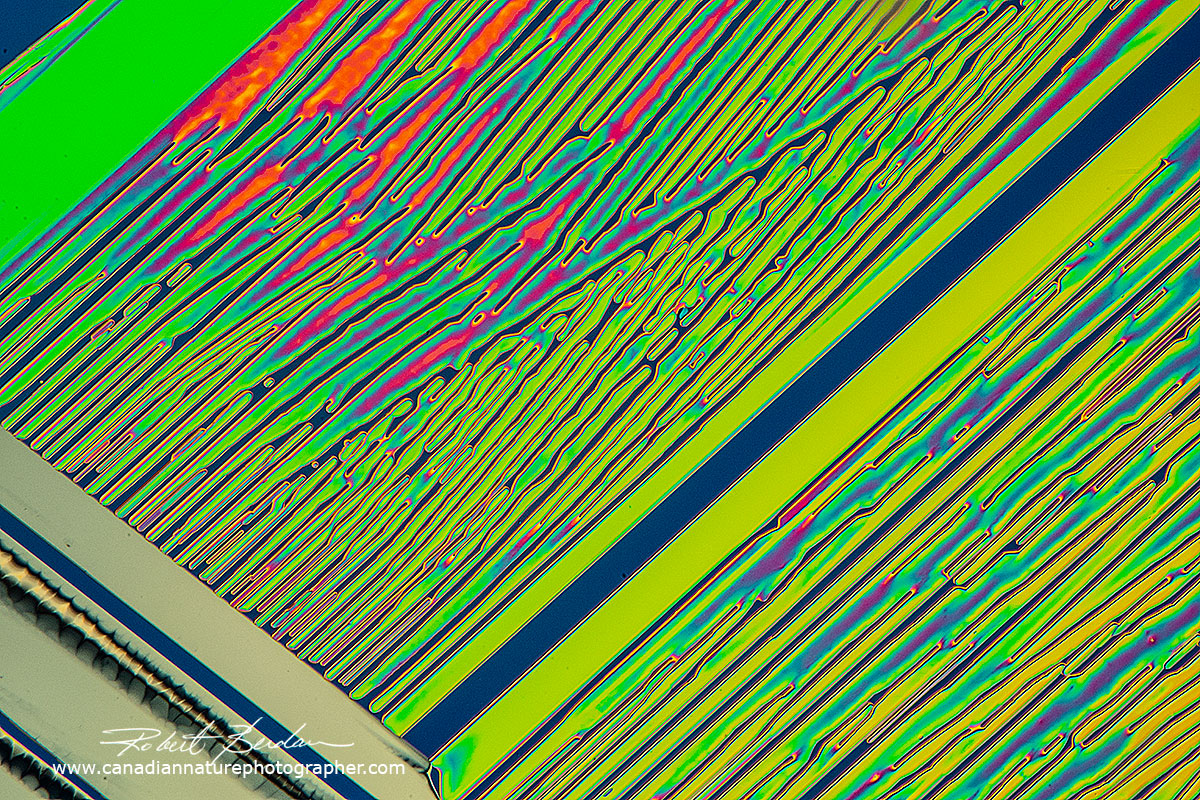
Above Urea crystals produced by melting 100X

Sulfamic acid crystals formed from a solution dried under a coverslip. I purchased the sulfamic acid at Home Hardware, it has a melting point of 205°C. Sulfamic acid is used as an acidic cleaning agent to remove rust and lime-scale. DIC microscopy 400X, focus stack of images.

Sulfamic acid produced using the melt method - polarized light microscopy 100X
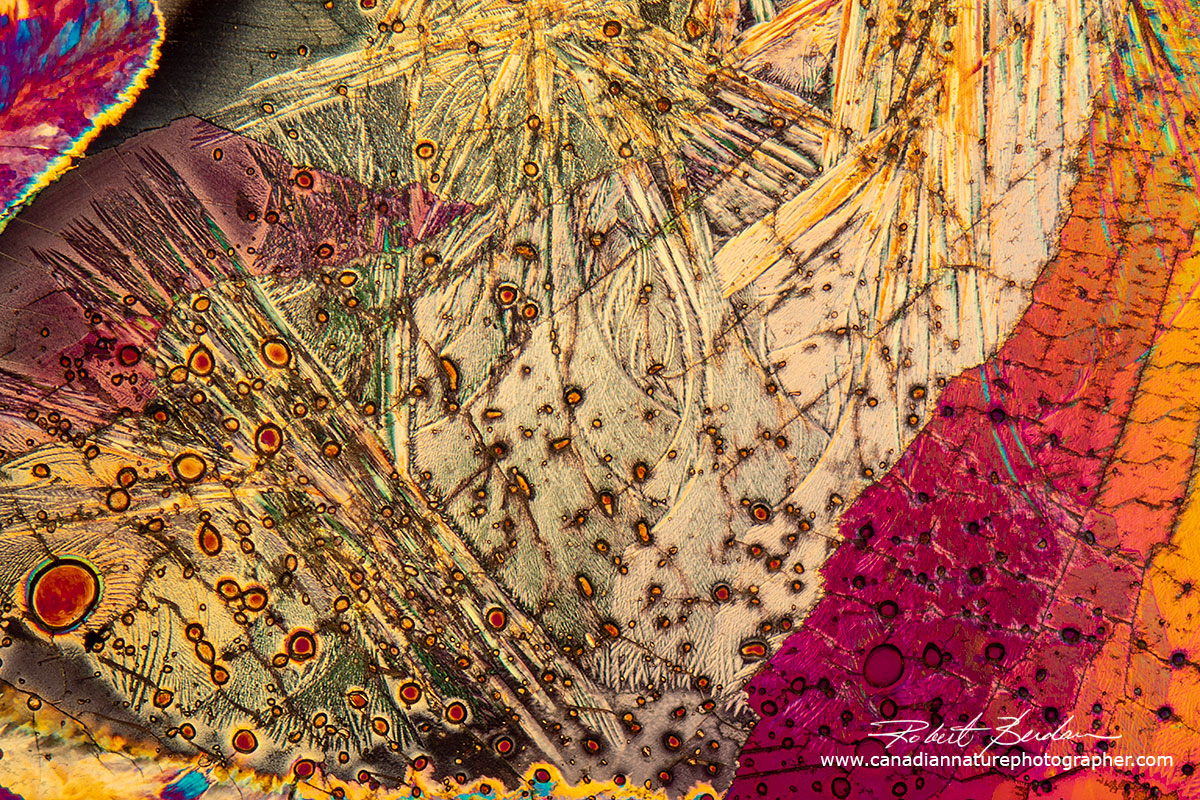
Above sulfamic acid crystals formed by the melt method.
Beer and Alcoholic Drinks
The late Dr. Michael W. Davidson was one of the first to popularize photomicrography of drinks using polarized light and started a web site Bev Shots to sell the images to help fund his research. While he provided some basic descriptions about his techniques he stated that he used proprietary crystallization methods and on his Molecular expressions web site (see links at end) he provided some clues. Anyone that has tried to create crystals from alcohol knows it is not easy. Some drinks like Vodka simply evaporate leaving little behind. Getting beer to crystallize is easy if you freeze it, but the crystals produced by freezing are not that unique in my opinion. Getting the components within beer to crystallize, even after concentrating the beer, can take weeks and requires some luck. Many of the beers I have tried never produced crystals. Seeding, adding salt seems to help in some case. Some of my successes are shown below.
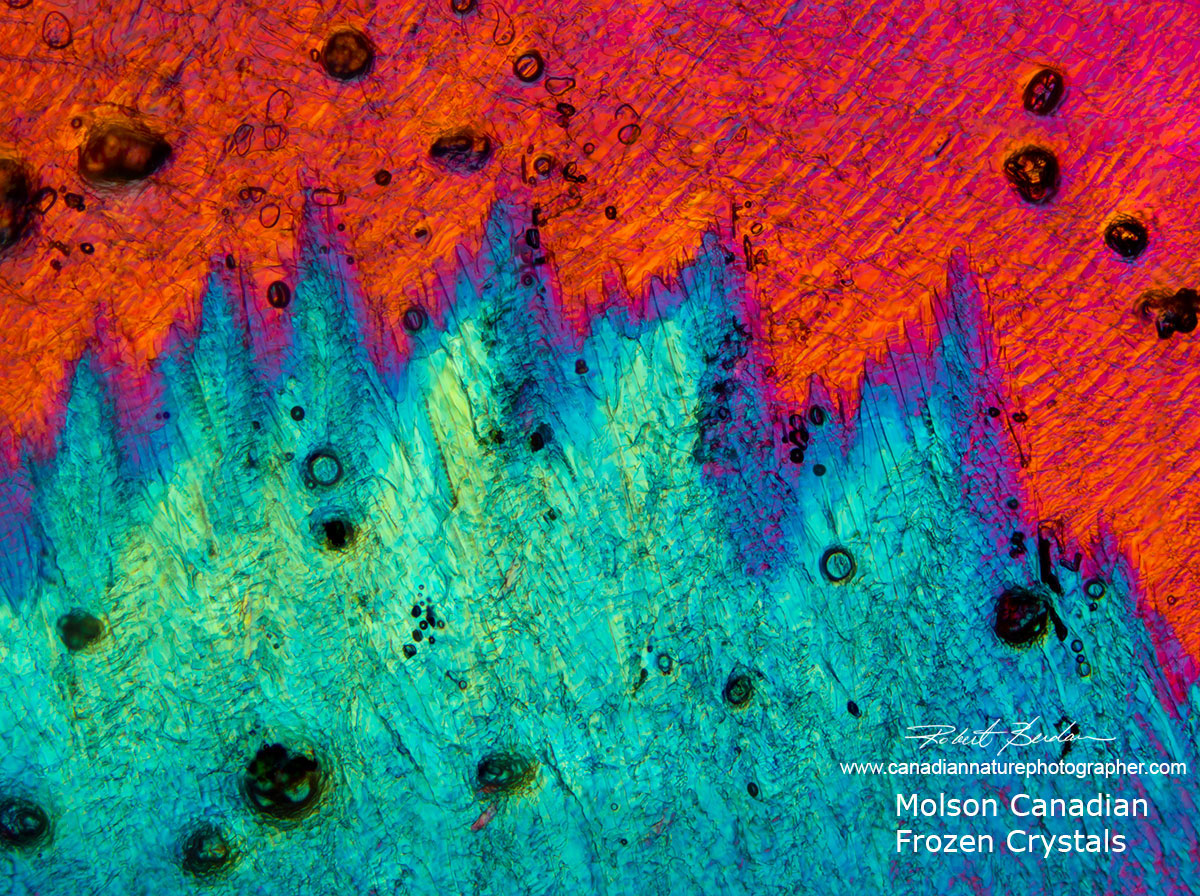
Molson Canadian beer crystals formed by freezing the beer and viewing by DIC microscopy.

Molson Canadian beer frozen and examined with DIC microscopy 50X.
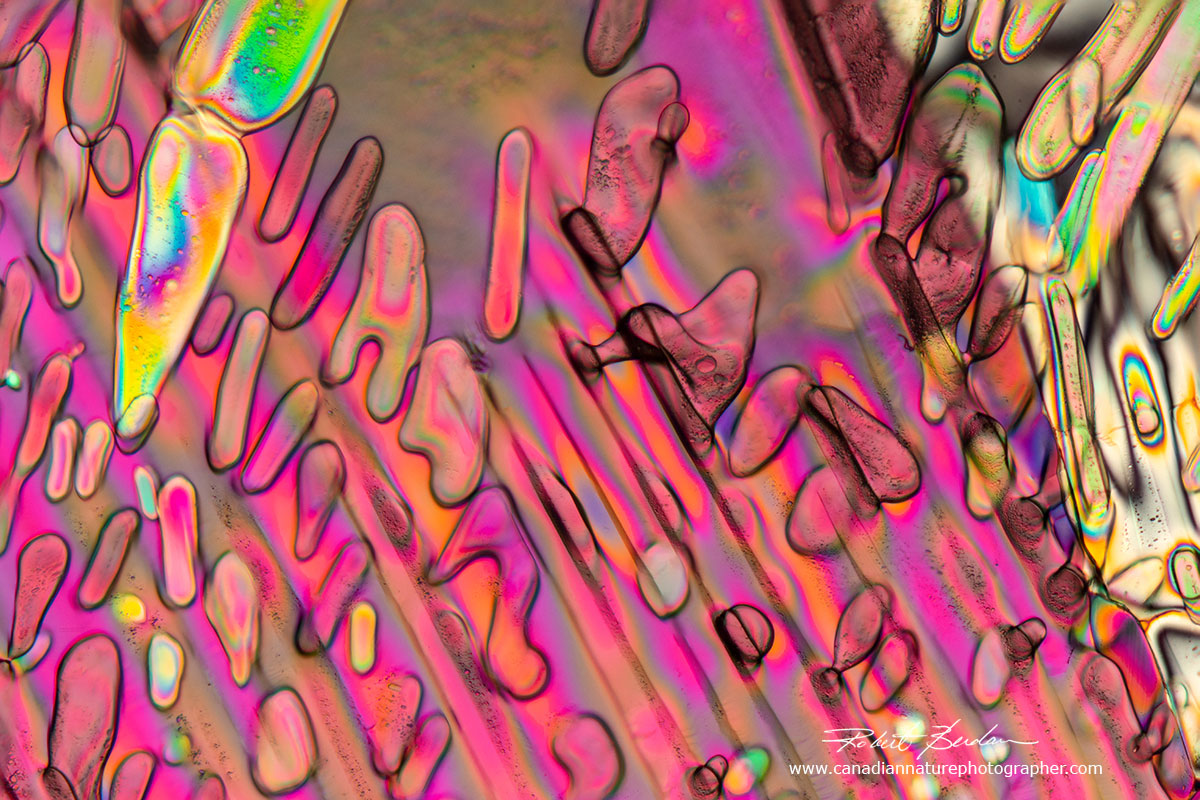
Crystals formed in beer frozen at -20°C 200X.
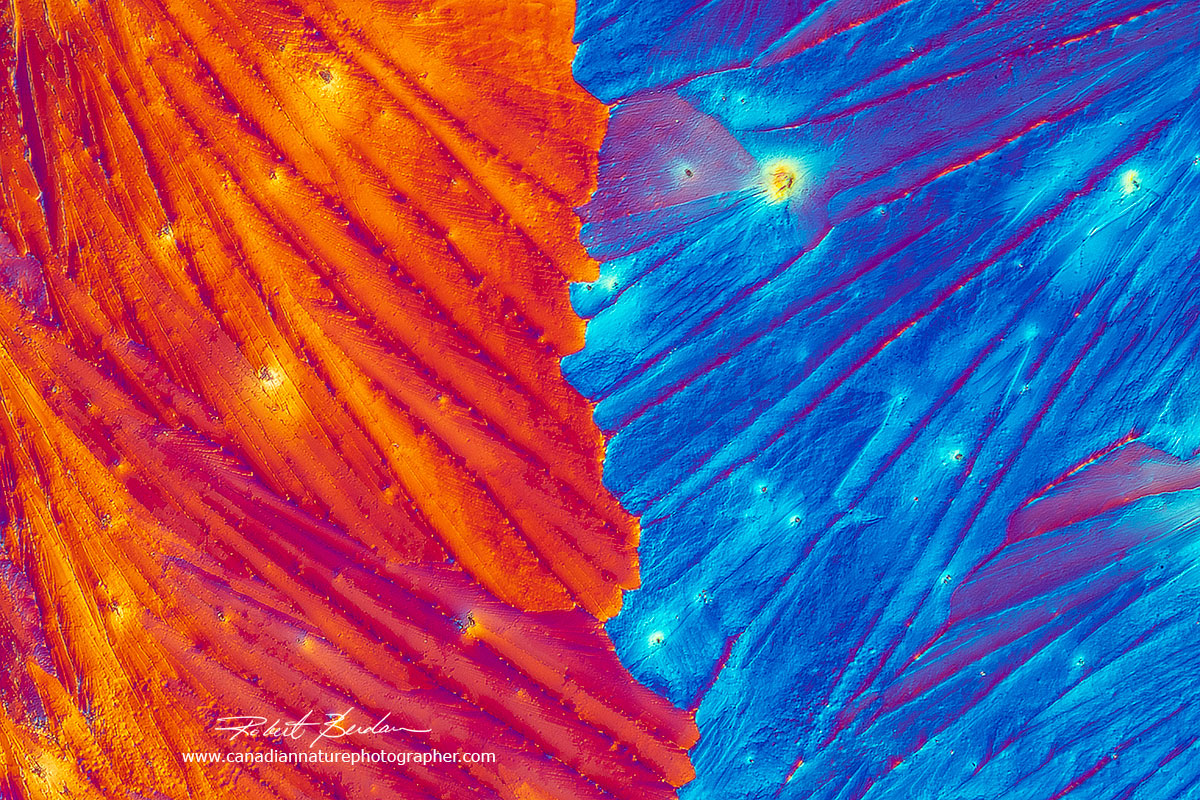
Beer crystals photographed with DIC microscopy

Corona beer crystals appeared after a few days - 400X.

Crystals from Corona extra beer - they were grown over a period of weeks under the coverslip.

Crystals in Corona Extra beer form feather shape crystals (most likely salt) which appear colourless in a polarized light microscope but by Differential Interference contrast microscopy I can create different coloured backgrounds and photograph a variety of crystal shapes. DIC 200X.
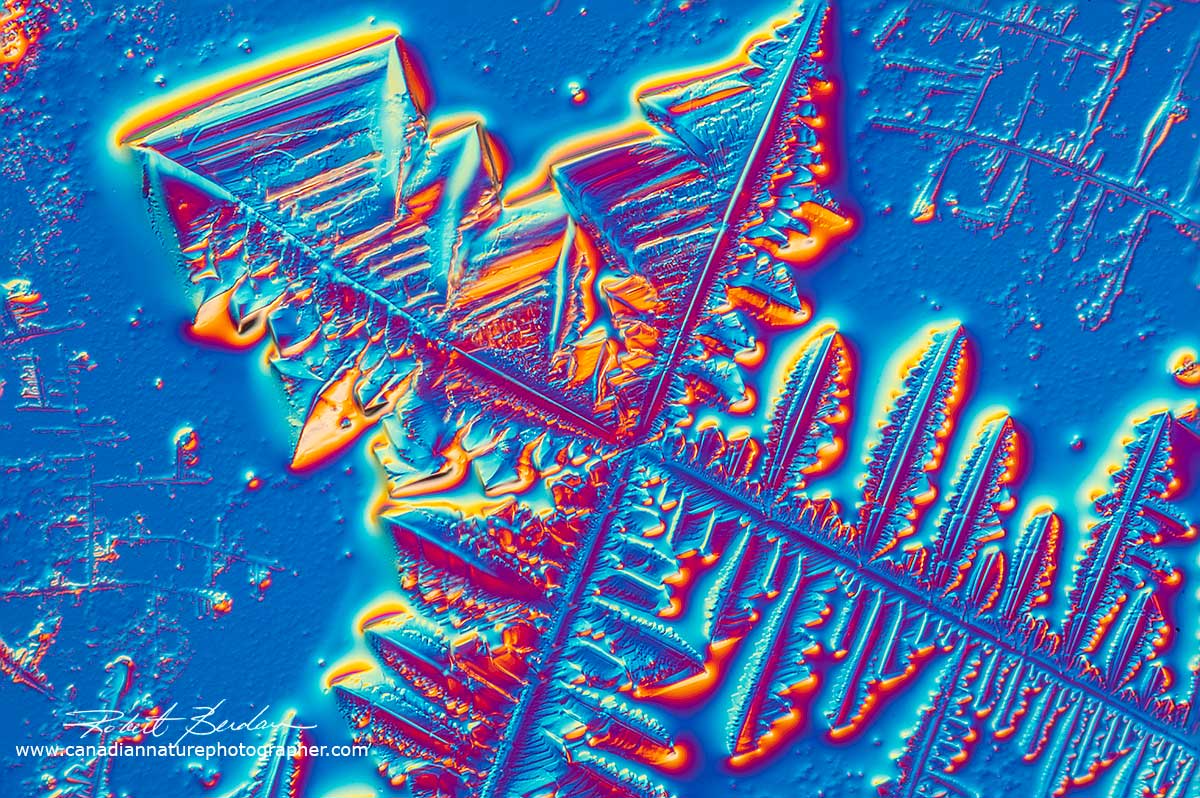
Captain Morgan Rum sample I seeded and photographed with Differential Interference microscopy. 400X.
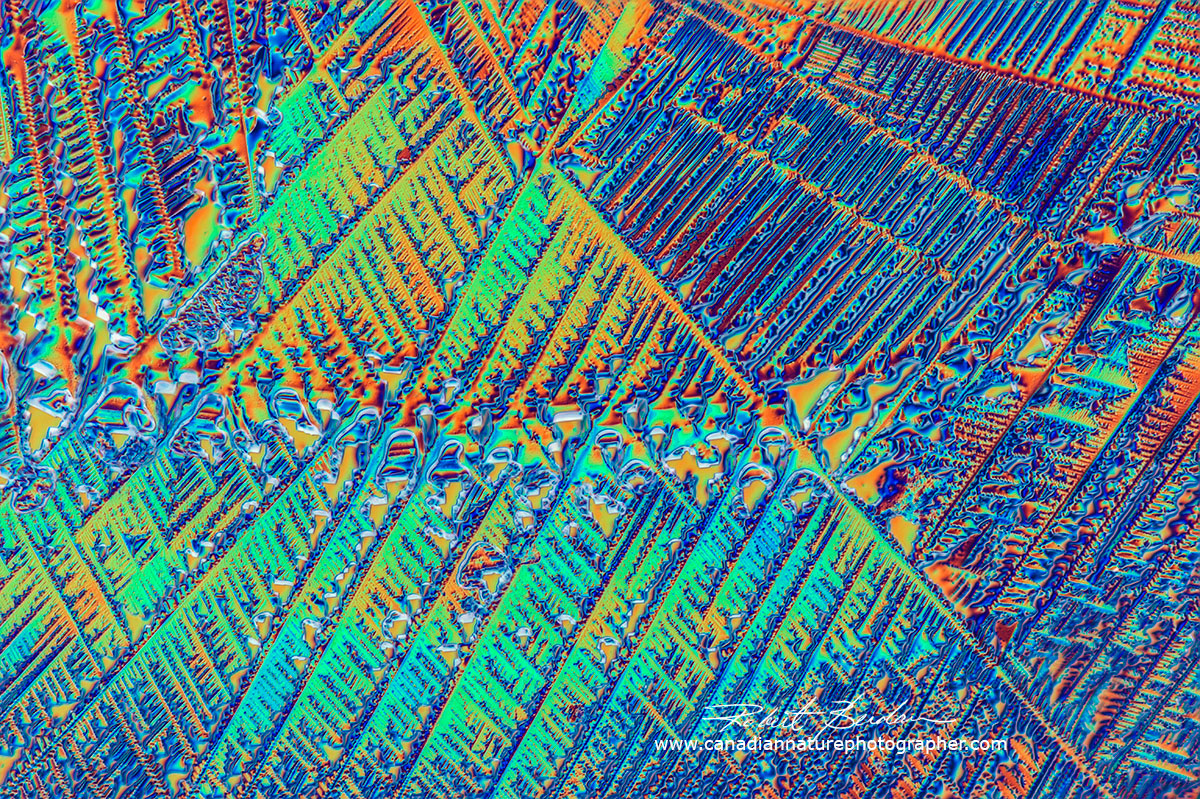
Corona Extra Beer Crystals using Differential interference Microscopy 200X.
In my experience getting beer and alcoholic drinks to crystallize is challenging. Concentrating the solutions by boiling then seeding with salts seems to help but it is not something you want to start out with. Other agents like Vitamin C, caffeine, citric acid, and Epsom salts are much easier to work with.
Caffeine
Caffeine is considered one of the world's most addictive drugs and an important commodity on world markets. Coffee boosts alertness, increases our reaction time by 3%, can increase our maximum oxygen capacity and boost our endurance. When viewed by polarized light microscopy it also spectacularly beautiful. When mixed with water it produces needle-like crystals, and when melted (melting point 235 °C) it produces map like crystals.

Caffeine crystals produced by the melt method and viewed by polarized light microscopy 100X.

Above - Map like crystals of caffeine produced by melting the powder on a microscope slide -100X.
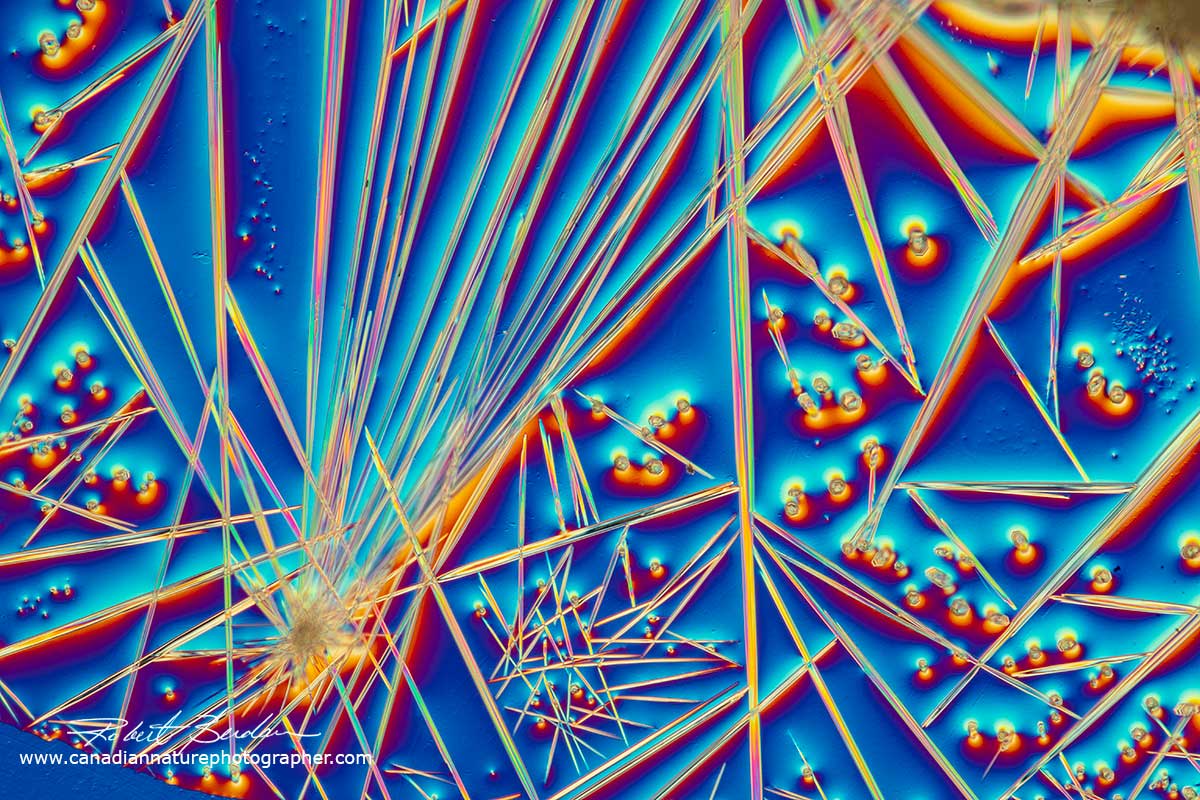
Caffeine crystals from energy drink (Red Bull) viewed by DIC microscopy 100X.
Dopamine Crystals
Dopamine is a neurotransmitter that has important functions in the human body and brain. It has many synonyms e.g. 3-Hydroxytyramine. It plays a role in reward-motivated behaviour which increase the levels of dopamine in the brain. I had some powder left over powder from when I was doing neuroscience research.
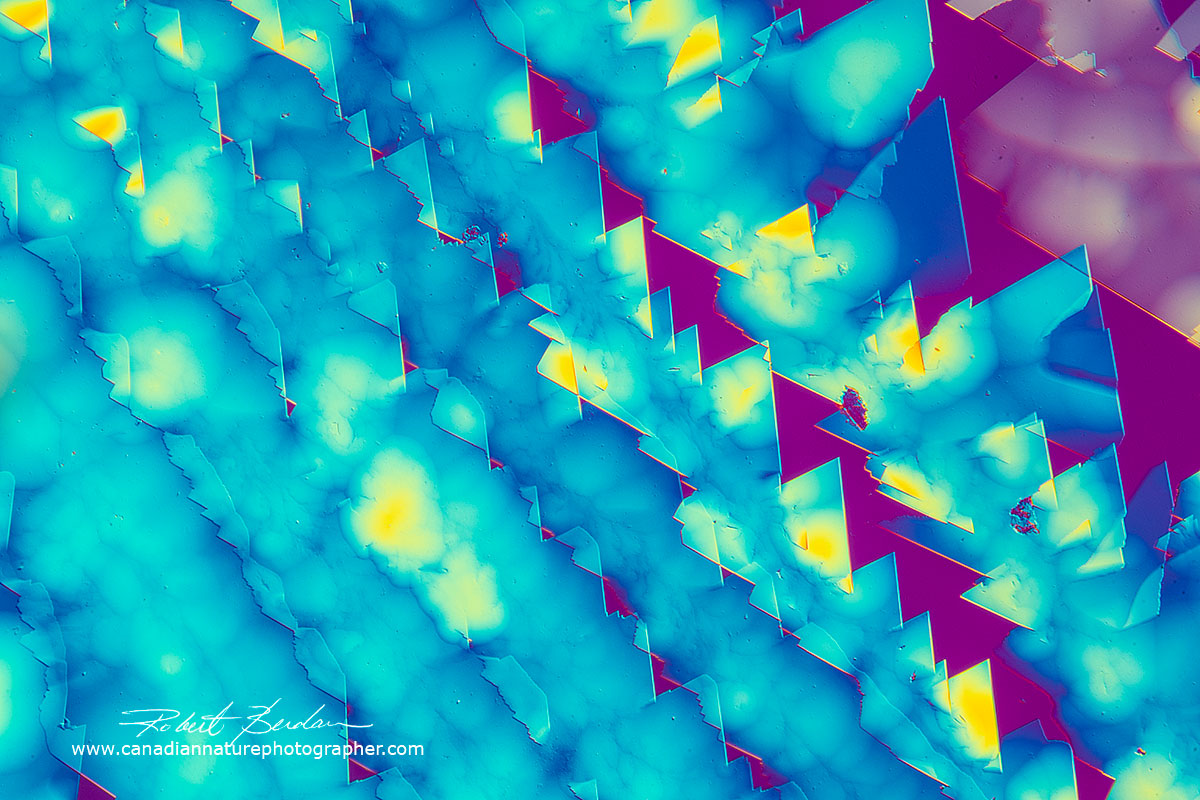
Dopamine crystals dissolved in water and air dried 100X DIC microscopy - 100X
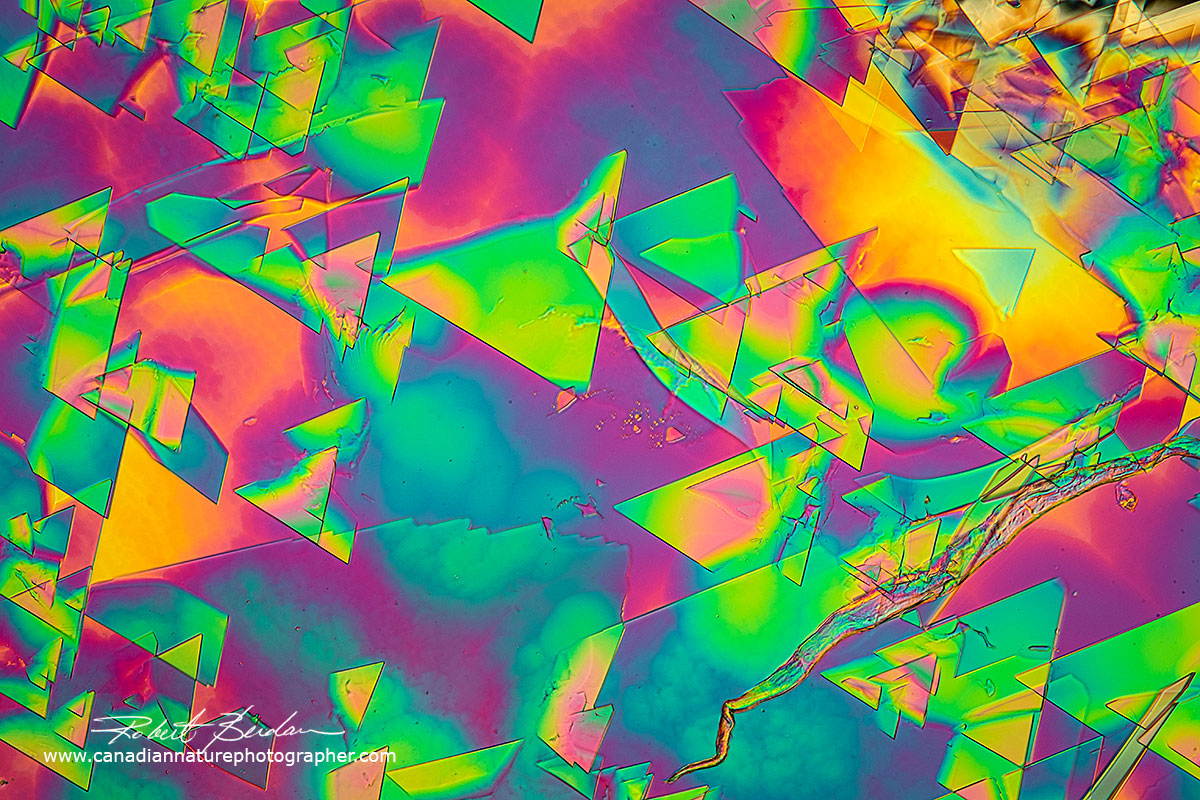
Dopamine in water air dried polarized light microscopy - 100X
Miscellaneous Crystals
Lysine - an amino acid dissolved in deionized water and viewed after air drying by Differential interference microscopy

Sulfonic acid crystal in water air dried and viewed by Differential Interference Microscopy 400X

Potassium citrate crystals dissolved in water and air dried polarized light microscopy 100X

Benzoic acid crystals polarized light 100X. Benzoic acid is a fungi-static compound that is widely used as a food preservative. It is a nitrogen binding agent and its melting point is 122°C.
Summary
Crystals viewed by polarized light microscopy and differential interference produce some of the most colourful images that occur in nature. Soap films also have beautiful colours produced by light interference. Anyone can do this type of photography if they have access to a light microscope, some polarizers, and a method to attach your camera to the microscope. For some tips on photomicrography see my article on this site. Of course there are many other things you can view and photograph with a microscope, many of them are alive and move quickly, but crystals are one of the most satisfying subjects. Photomicrography of crystals is a great outlet for one's creativity and the abstract patterns are mesmerizing. If you live near Calgary, I would be happy to teach you how to do this type of photography in my home microscopy lab. I have adaptors for Nikon and Canon Cameras or you can use one of my cameras to take the photos and then take the images home with you on a USB stick. I also provide a service to photograph microscopic items - see my other commercial photography web site - Science & Art. If you have questions about photomicrography I will do my best to try and answer them. RB

Composite image of polarized light photomicrographs showing several different crystals.I plan to test many more chemicals in the coming year and follow up with more pictures and movies.
Download movie of Crystals & Music mp4 195 MB 1920 x 1080 - can be used for teaching in a classroom or personal viewing or you can watch the video on Youtube Made with Window Movie Maker shown above. For commercial use of images please contact me.
See more crystal photomicrographs on my Gallery
Or watch Video on YouTube https://youtu.be/riDgQarL44A - you can also view full screen 1920.
Most of the crystals are of Vitamin C there are few other crystals sulfamic acid and a sports drink.
Interested in Purchasing Science Images as Art?
All of my images can be purchased for personal or commercial use - see my price list for personal use. For commercial use price depends on whether you need exclusive rights and what the image will be used for. If you are interested please contact me and ask about specific photos or custom images. I offer photomicrography services on my commercial web site Science & Art.
References and Links
Protein Crystallization Wikipedia - good overview
Photography of Beer and Cocktails through the microscope - Molecular Expressions
Polarized Light Microscopy - Specimen Preparation - Molecular Expressions
Tribute to Dr. Michael W. Davidson
Molecular Expressions - The Vitamin Gallery by Dr. Michael Davidson
M.W. Davidson - Fascinating Photos with a Simple Microscope - PDF
Your Tears are as unique (and Beautiful) as Snowflakes
Brian Johnston - A Gallery of Ascorbic Acid Photomicrographs
Justin Zoll - Beautiful Microscopic landscapes with Polarized Light Microscopy
Manfred Kage - German microscopist photographing crystals for decades
K. Libbrecht worlds authority on snowflakes - check out snowcrystals.com
D. Komrechka Sky Crystals - Photographing and Understanding Snowflakes - article on this site
S. Santos (2015) Crossing borders: the path of photomicrography towards artistic recognition.
J. Desarnaud et. al. 2018 Hopper Growth of Salt Crystals. J. Phys. Chem. Lett. 9: 2916 - PDF
The influence of sodium chloride on the crystallization rate of gypsum
A. McPherson (2001) A comparison of salts for the crystallization of macromolecules - PDF
A. McPherson and J. A. Gavira (2014) Introduction to protein crystallization - PDF
A. McPherson and L.J. DeLucas (2015) Microgravity protein crystallization - PDF
A. McPherson (1976) Crystallization of Proteins from Polyethylene Glycol. J. Biol. Chem. 251:6300 6303 - PDF
M. Yamanaka et. al (2010) Optimization of salt concentration in PEG-based crystallization - PDF
T. Bergfors (2003) Seeds to Crystals. J. Structural Biology 142:66-76 - PDF
L. Modderman (2001) Micro-crystals in Polarized light, and How to Grow them
A. Arslantas et al (2004) Crystal Habit Modifications of Vitamin C due to Solvent Effects. Turk. J.Chem 28: 255-270 - search google for PDF
R. L. Howey (2019) The Lust for Fine Microscopes -
view photomicrographs of crystals
R.A. Carlton Polarized Light Microscopy (2011)- General Overview - Download PDF
Polarized Light Microscopy Basics by Olympus
Dr. P. Wasilewski - Frizion web site that sells pictures of polarized ice photomicrographs
NASA Scientist looks at Olympic Ice in a Frozen light - Dr. P. Wasilewski
Related Microscopy Articles by Robert Berdan on this web site
1. Photomicrography of Hydra - a model for studying regeneration and aging
2. Photographing Daphnia
3. Photographing Gastrotrichs
4. Photographing Rotifers
5. Photographing Ciliates
6. Photographing Stentors - A Large Unicellular Protozoan (ciliate) living in Freshwater
7. How to Collect and Photograph Water Bears (Tardigrades).
8. Tips on How to take Better pictures with a Microscope
9. Microscopic Pond Organisms from Silver Springs Calgary
10. Microscopic Life in Ponds and Rainwater - Pond Scum I
11. Photographing Microscopic Plant and Animal Life - Pond Scum II
12. Photomicrography and Video of Protozoa, Volvox and Rotifers
13. Home Microscopy Laboratory for Photomicrography
14. The Art & Science of Photomicrography with Polarized Light
15. Photographing Through a Microscope Photomicrography - Inner Space
16. Focus Stacking comparing Photoshop, Helicon Focus and Zerene
17. Rheinberg Filters for Photomicrography
18. Scanning Electron Microscopy - Photography
19. Photomicrographs of Diatoms from 1877 by John T. Redmayne
Authors Biography & Contact Information
 Bio: Robert Berdan is a professional nature photographer living in Calgary, AB specializing in nature, wildlife and science photography. Robert retired from Cell\Neurobiology research to take up photography full time years ago. Robert offers photo guiding and private instruction in all aspects of nature photography and Adobe Photoshop training - including photomicrography, macrophotography.
Bio: Robert Berdan is a professional nature photographer living in Calgary, AB specializing in nature, wildlife and science photography. Robert retired from Cell\Neurobiology research to take up photography full time years ago. Robert offers photo guiding and private instruction in all aspects of nature photography and Adobe Photoshop training - including photomicrography, macrophotography.
Email at: rberdan@scienceandart.org
Web sites:
www.canadiannaturephotographer.com
www.scienceandart.org
Phone: 9 am -7 pm MST (403) 247-2457.