
Didinium nasutum versus Paramecium caudatum
Predator and Prey
by Dr. Robert Berdan
May 25, 2021

Didinium nasutum (left) is a one celled ciliate living in lakes and ponds and preys upon other ciliates in particular Paramecium caudatum (right) 200X differential interference microscopy (DIC).
Introduction
Watching one animal hunt and prey on another is educational. Single-celled ciliates are often used as models to study predator prey relationships (L.S. Luckinbill 1973, A. Baneri et al. 2015). Didinium nasutum preys on a variety of single-celled ciliates, but seems to prefer Paramecium species. I visit ponds regularly once the ice melts in early April in Calgary and until they freeze again in late October. Paramecium are common ciliates but I have never found Didinium in the wild. Most researchers purchase Didinium from a scientific supply company. I ordered some Didinium from Boreal labs along with some other protists while the ponds were still frozen in March of 2021. I wanted to photograph the interactions between Didinium and other ciliates and observe their behaviour in my home microscopy laboratory. In this article I will share some of my observations, images and movies of these astonishing animals as they interact in the micro-world.
Didiniun nasutum

Didinium nasutum viewed by DIC microscopy 400X. Note the central C shaped nucleus, thread- like structures in the seizing organ (toxicysts, and pexicysts) on the apical end and the two tufts of cilia (pectinelles) that surround the organism.

Didinium viewed by phase contrast microscopy 400X - note the C shaped nucleus and some ciliary bands.
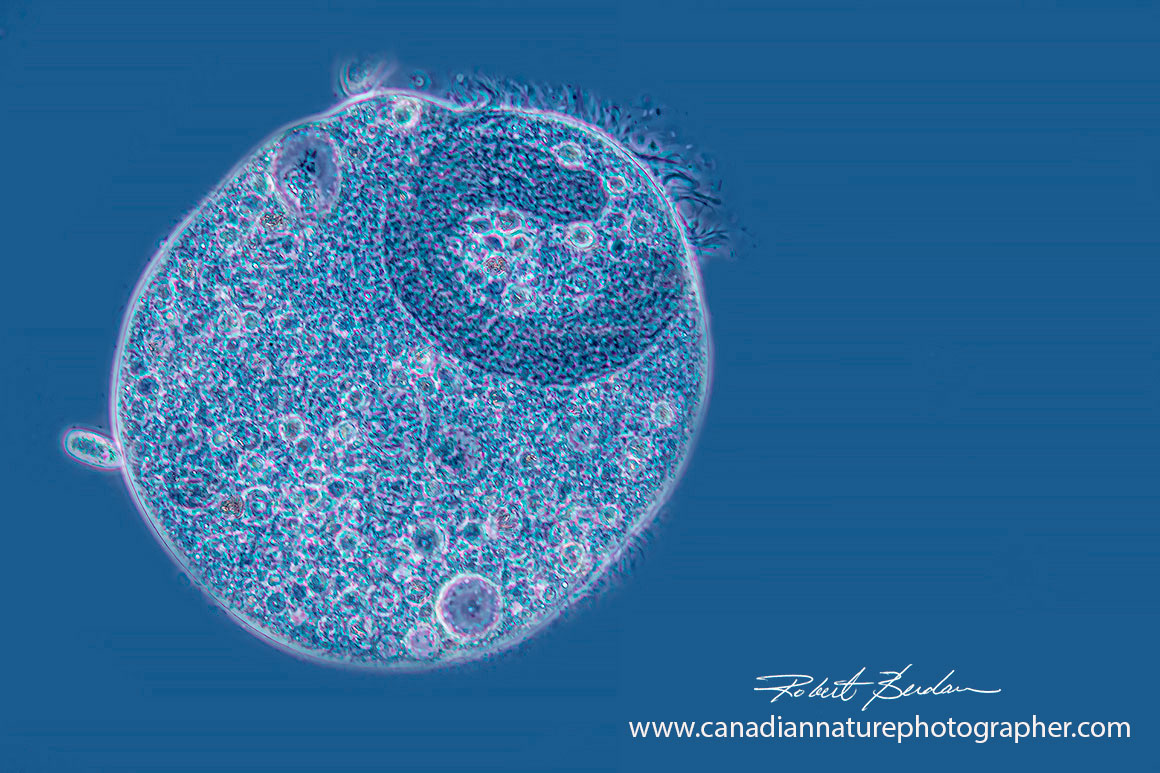
Didinium by Phase contrast microscopy - C shaped nucleus near the top 200X.
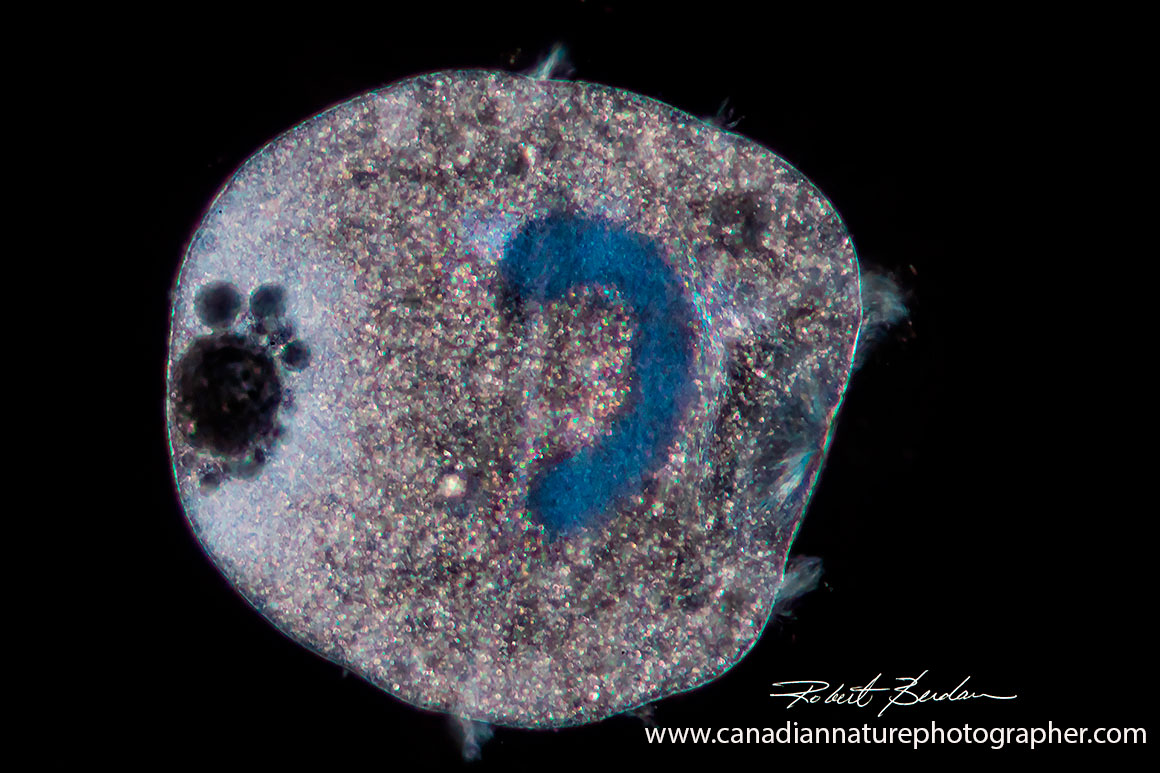
Didinium observed by darkfield microscopy shows the C shaped macronucleus, posterior contractile vacuoles and pectinelles 400X.
Didinium is a medium-to small ciliate about 50-150 microns (1 micron = 0.001 mm) in length and is found in fresh and brackish water. So far I have not found Didinium in ponds or lakes in Alberta but they have been found in the great lakes according to aquatic biologists. These barrel-shaped ciliates have two circular rows of ciliary bands used for locomotion. They have a rounded conical end (the seizing organ or proboscis) that is supported by a palisade of rods (microtubules) called nematodesmata. Didinium has an apical cytostome (mouth) characteristic of other ciliates like Lacrymaria, Spathidium and Dileptus. Didiniums' macronucleus is long and C shaped and is sometimes twisted into a figure eight. Didinium has a contractile vacuole on their posterior end along with an anal aperture. When food is limiting Didinium form cysts which can be viable for up to 10 years. See H. Wessenberg and G. Antipa (1970) to view transmission and scanning electron micrographs of Didinium and the seizing organ (Proboscis).
Didinium feeds primarily on Paramecium species (P. caudatum, P. aurelia, P. bursaria, P. multimicronucleatum) but it also feeds on other ciliates like Colpoda and Frontonia. Balbiani (1873) was the first to study feeding habits of Didinium. Didinium does not feed on the ciliate Spirostomum (unless cut into pieces) or attach to Euglena and rotifers (S.O. Mast 1909). Spirostomum is a long slender tube-shaped ciliate that can reach 1 mm or longer. It seems the large size of this organism is a deterrent to Didinium. I observed Didinium bump into Spirostomum numerous times and it never tried to attach itself. It is interesting that Didinium makes a quick decision to attach or go on its way after bumping into an organism. I often saw Didinium bump into Paramecium without attaching as well. Didinium must have some sort of recognition mechanism to identify edible and inedible organisms, but how this is done is unknown.
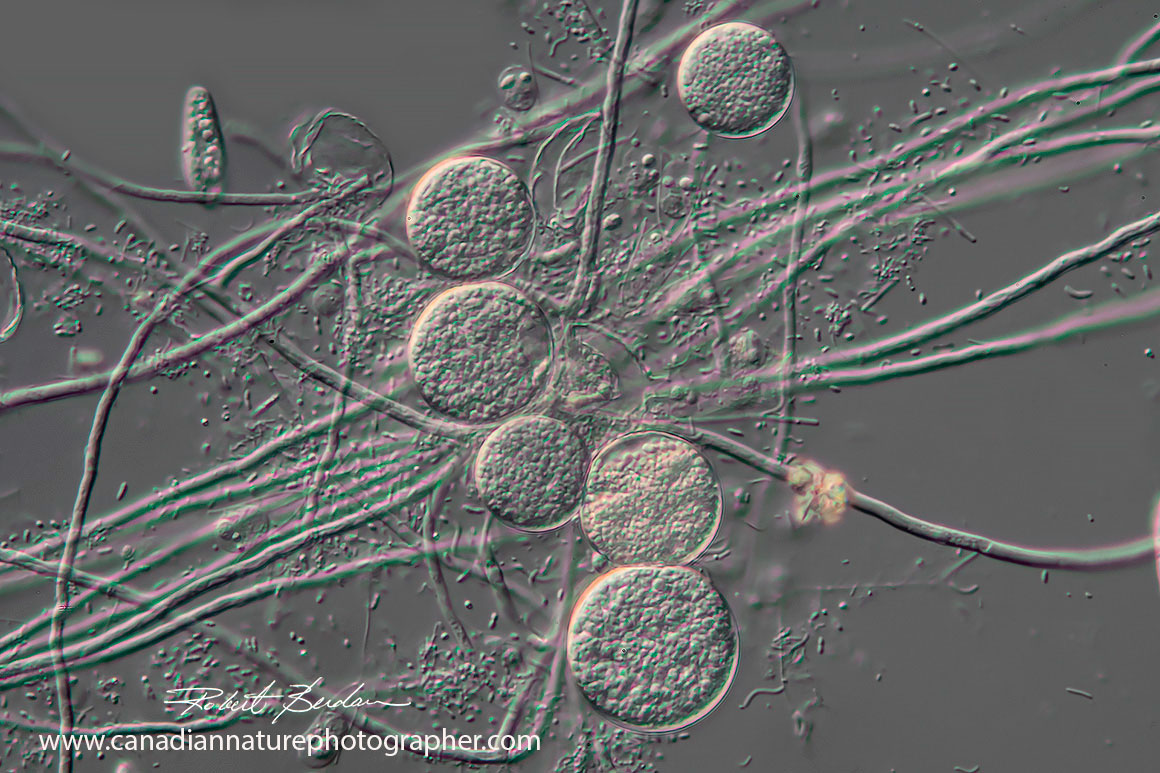
Cysts found in the Didinium container from Boreal Science which I believe are those from Didinium nasutum.
Other microorganisms in the Jar with Didinium
I purchased Didinium from Boreal labs and when the samples arrived I found primarily a few rotifers; Euglena, Paramecium, Blepharisma sp and cysts in the sample bottle on the first day of arrival. The next day Didinium were visible - the sample bottle was cooled during transport. S.O. Mast (1909) reported that Didinium were induced to come out of their cysts by adding water. I sampled the jar and viewed drops on a microscope slide to observe and photograph them. Didinium was abundant for about four days then they seemed to disappear even though there were still Paramecium in the jar. There were also other protists including Euglena, Blepharisma (pink-red ciliate) and rotifers in the jar - see pictures below.

At the top of the picture is the ciliate Blepharisma americanum which is pink in colour with 4 macronuclei. Below it is a Paramecium caudatum. Didinium often contacted Blepharisma but never attacked it. Blepharisma exhibited no change in behaviour when it was in contact with Didinium. It is possible that the red pigment granules made up of blepharismin are protective (T. Harumoto et al. 1998). I did not see the release of any extrusomes when contacted by Didnium. Extrusomes are membrane-bound structures released by some ciliates and flagellates in response to physical stimuli or when being attacked. DIC microscopy 200X.

Blepharisma sp is red to pink in colour due to granules of the pigment blepharismin. All members of the genus possess a long series of membranelles on the left side of the oral groove, and an "undulating membrane" (Wikipedia). There are about 40 species that vary in size and shape some are transparent. DIC microscopy 200X.

Paramecium caudatum by DIC microscopy. 200X

Paramecium showing rows of trichocysts (extrusome) just under the membrane. The trichocysts are protective but are not effective against Didinium. 600X DIC microscopy.
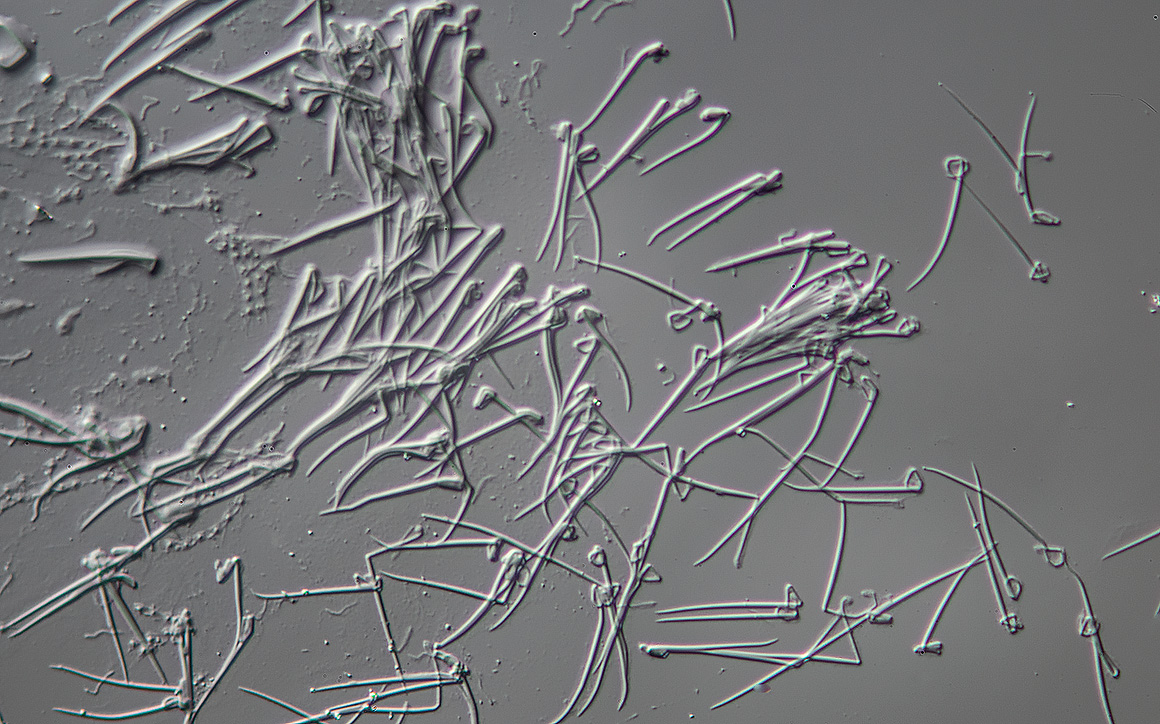
Trichocysts released by Paramecium were ineffective in deterring Didinium 400X DIC microscopy.

Euglena gracilis a single celled alga by phase contrast microscopy 1000X was present in the Didinium jar. Note the long flagellum.
Didinium Behaviour
Didinium is extremely active and seldom found at rest. Didinium is constantly rotating along its longitudinal axis, stopping, then turning as it searches for food. When it comes in contact with anything it does not eat it reverses the stroke of cilia, backs a short distance, turns to the right side and then proceeds. I saw Didinium bounce off Paramecium caudatum many times as if it didn't recognize it as food. I also watched Didinium make numerous contacts with Spirostomum and Blepharisma ciliates and yet it never tried to eat these organisms.
When Didinium attached itself to Paramecium there was a rapid extrusion of trichocysts from Paramecium (porcupine-like spines that seemed to have no effect). Didinium also released extrusomes called pexicysts and toxicyts though I was not able to see them. The pair struggled violently for a few seconds, then the Paramecium would become still and was pulled inside Didinium within 1-3 minutes. When trichocysts were released by Paramecium they were not effective in repelling Didinium. The events after Didinium captured a Paramecium were difficult to photograph due to the shallow depth of field with DIC microscopy so I recorded them with video and tried extracting some of the frames with partial success (see below).
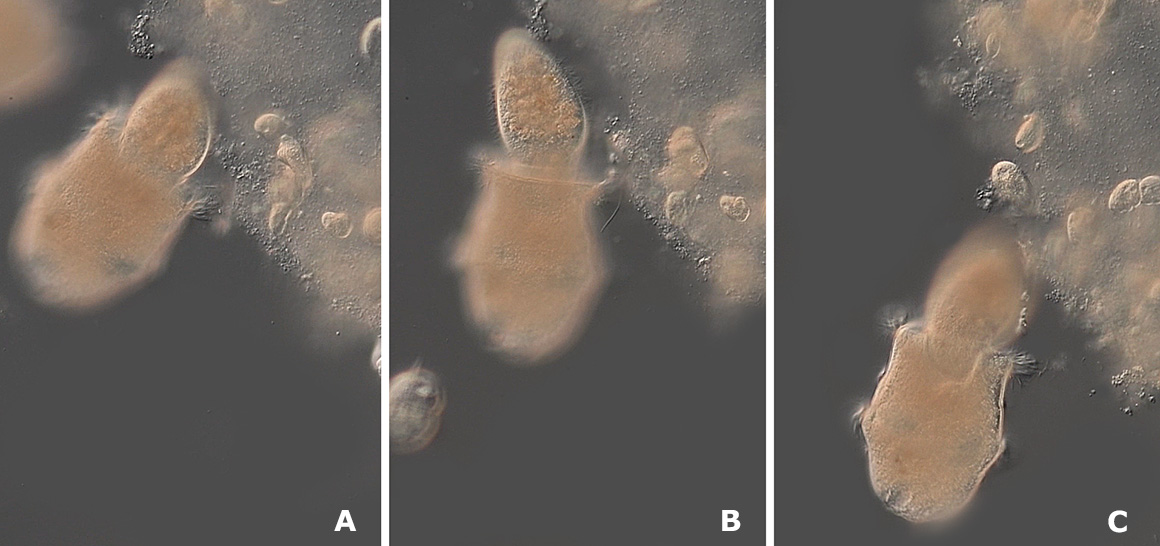
Three frames from a video after Didinium captured a Paramecium. DIC microscopy
When I mixed Didinium with Paramecium in a drop of water on a microscope slide Didinium behaved like bees around a disturbed nest moving in all directions while searching. Paramecium also seemed to swim faster and would "park" in debris as if to hide. Since neither species can "see" and detect each other visually they would often pass each other within less then a body length. Sometimes a Paramecium would stop suddenly when approached by a Didinium and it seemed as if the Paramecium could sometimes detect the Didinium approaching, perhaps by some chemical scent. Watching the Didnium hunt while Paramecium tried to avoid Didnium was fascinating. I would watch them for several hours at a time. Some ciliates like Spirostomum and Blepharisma behaved normally often making contact with Didnium but were not attacked. Sometimes when Didnium contacted the side of Paramecium, it appeared to pierce and grab hold of it and after a second or two the Paramecium appeared paralyzed and the Didinium would swallow it quickly - often in less then 60 seconds.

Above is a Paramecium that escaped capture after passing between between two Didinia. Note that this was one of the smaller Paramecium. Paramecium were often 1.5 to 2X the size of the Didinium. 200X DIC.

Larger Paramecium that was captured by the Didinium. DIC microscopy. This picture shows the variation in size of the Paramecium compared to Didinium.
What happens when Didinium Contacts Paramecium

An illustration showing Didinium nasutum consuming a Paramecium.
P Paramecium, D Didinium, CV contractive vacuole, c bands of cilia, n nucleus, t trichocysts, s seizing organ from from S.O. Mast, 1909.
When Didnium bumps into Paramecium often times it would back up, turn and be on its' way, especially if it contacted the anterior or posterior end of Paramecium. If Didinium contacted the side of a Paramecium, Didinium would direct the "seizing organ" on its apical end and appeared to push the apical end and revolve. The Paramecium would release trichocysts (fine fibers), that were ineffective in protecting it. The seizing organ seem to latch onto the Paramecium and pull it inside. Within a few seconds the Paramecium was being drawn into the Didinium and was completely enclosed within 1-3 minutes. According to S. O. Mast (1909) Didinium feeds about every 3 hours and divides about every 8-12 hours whether it fed or not. Some Didinium can consume Paramecium 12X their body size (S.O. Mast, 1909).
When a Didinium contacts a Paramecium, it discharges 2 kinds of extrusive organelles - short pexicysts which attach to the surface of Paramecium but do not penetrate and longer toxicysts that do penetrate Paramecium and the proximal ends remain embedded in the Didinium. Cytoplasmic streaming pulls the discharged toxicysts inward and draws Paramecium inside the Didinium (H. Wessenberg and G. Antipa, 1970). I could not see Didinium extrude toxicysts though I did see trichocysts released by the Paramecium. Presumably the toxicysts act like a harpoon to attach to the Paramecium. The toxicysts in the seizing organ may carry some kind of toxin because the prey stops swimming within seconds,and lysis and vacuolation is seen by transmission electron microscopy at the point of contact. The Paramecium also dies even if it is not swallowed (Wessenberg and Antipa, 1970).

Spirostomum sp is a long tubular-shaped ciliate (Heterotrich) that Didinium often contacted but would not attack. If Spirostomum is cut into shorter pieces Didinium will then consume the parts (J. Berger 1979). If ciliates like Paramecium bursaria with symbiotic algae (e.g. Chlorella) came into contact with Didinium it was avoided suggesting that the symbiotic algae discouraged predation by Didinium.

Above Didinium separated after being joined end to end for over 40 minutes - undergoing cell division. DIC microscopy. This behaviour was observed by S.O. Mast (1909) and would sometimes include more than 2 animals.

Picture of two Didinium. One animal in the upper left has 4 rows of pectinelles (cilia) and is in the beginning stages of cell division. DIC microscopy 200X .
Fluorescence Microscopy
I examined both Didinium nasutum and Paramecium caudatum using fluorescence microscopy after staining them with 0.001% solution of Acridine orange for a few minutes.
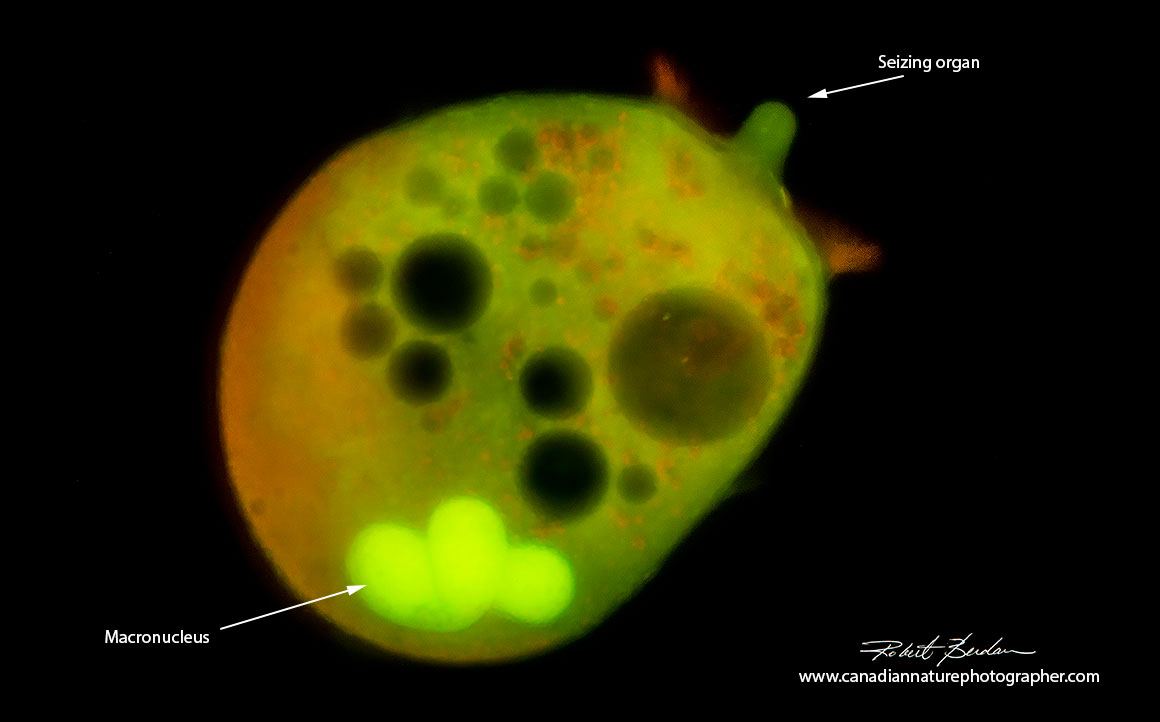
Didinium nasutum after staining with Acridine orange (0. 001% w/v) Fluorescence microscopy. Acridine orange stains DNA green, RNA red and lysosomes orange - 100X.

Didnium and C shaped nucleus Acridine orange and Fluorescence microscopy.

C shaped macronucleus of Didinium after staining cells with Acridine orange. The dye makes some ciliate membranes fragile so that when the organisms are centrifuged the nuclei escaped the cell membrane and become separated. Fluorescence microscopy 400X
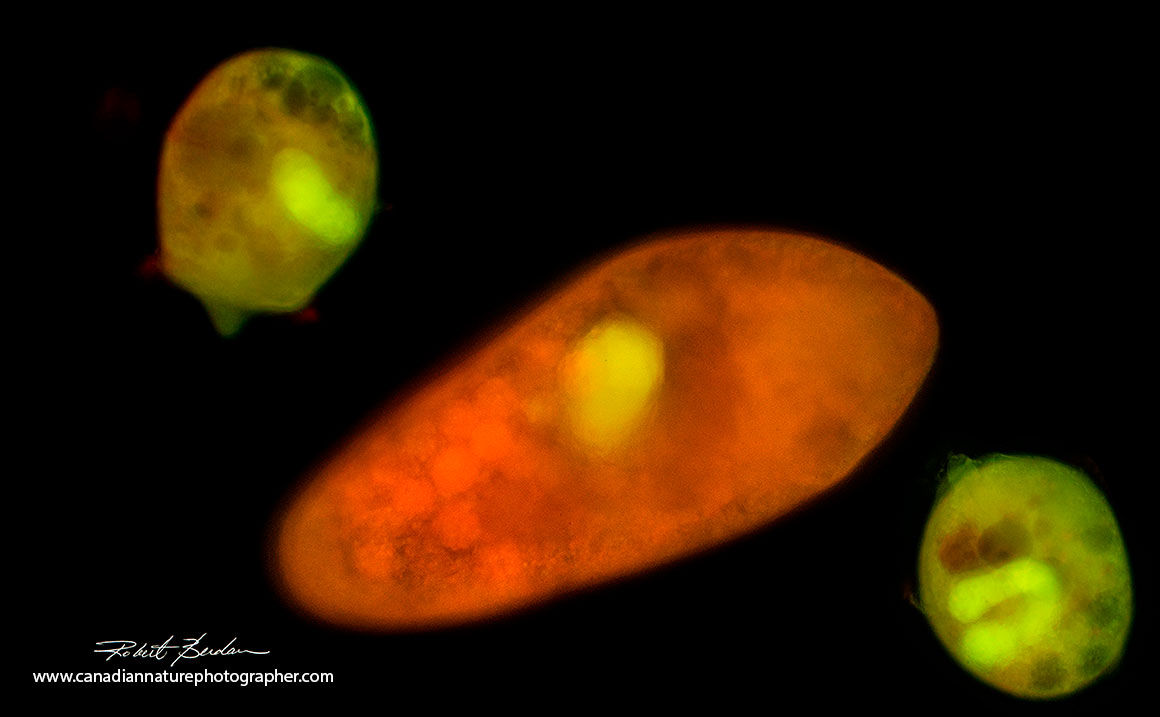
Several Didinium nasutum surrounding a Paramecium caduatum after staining with Acridine orange Fluorescence microscopy 100X.
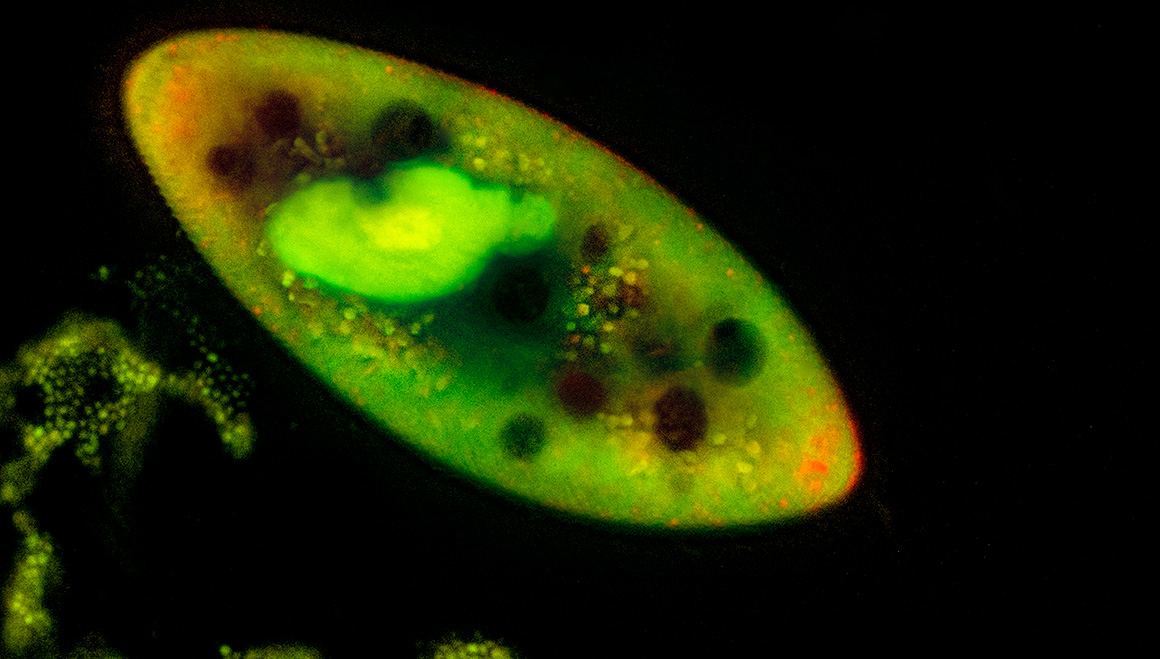
Paramecium stained wth Acridine orange and viewed by fluorescence microscopy 200X
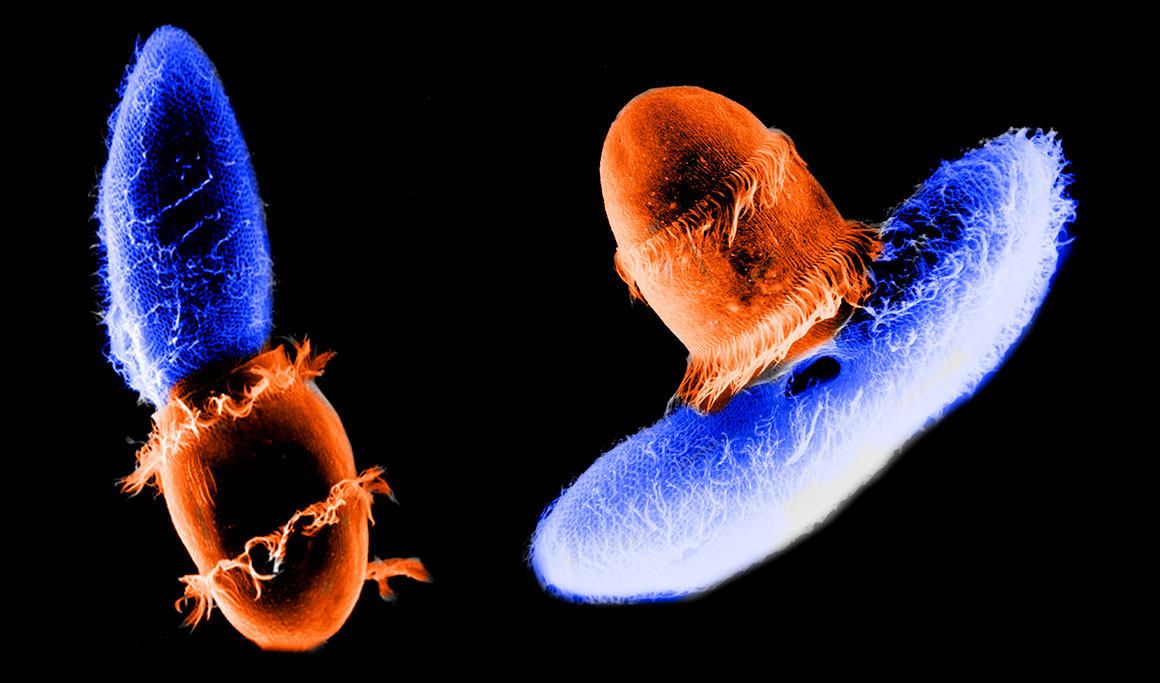
Coloured scanning electron micrographs of Didnium (orange) feeding on Paramecium (blue) photo by Gregory Antipa (San Francisco State University) and hand coloured in Photoshop.
Movie
Didinium nasautm feeding on Paramecium caudatum
Watch on Youtube: https://youtu.be/wx-FZjf-nc4
Population Studies of Didnium and Paramecium
In model predatory scenarios using Didinium and Paramecium it was found that Paramecium were totally consumed within days and Didinia subsequently starved if more paramecium were not added. Scientists hypothesized that the prey needed a refuge to be exempt from predation. When methyl cellulose was added to the water to slow down the Didinium and Paramecium, the two populations oscillated a few times before they disappeared (L.S. Luckinbill 1973). Luckinbill went on to show that if the bacteria the paramecium were fed was reduced in number it reduced the number of Paramecium which permitted them to oscillate in numbers with Didinium. Coexistence can therefore be accomplished in a homogeneous environment if prey are able to achieve sufficiently low densities that predators are unable to capture them all and if there was some restriction on prey growth. In another study it was found that introducing a paramecium parasite (Holospora undula a bacterium that only infects Paramecium) it reduced Paramecium abundance and this indirectly reduced the number of Paramecium allowing both Didinium and Paramecium to coexist). The significance of this is that if a certain parasite is added as a biological control agent (e.g. for insect control) it can have unexpected consequences. Even the size of the Paramecium can influence the rate of capture by Didnium (A. Banerji et al. 2015).
Summary
Didinium preying upon Paramecium has been used to study predatory relationships, their small size, short life times and rapid reproduction make these animals good models for scientific analysis. Whereas Paramecium are commonly found in ponds around Calgary Didinium is not. However, Didinium can be easily purchased from Biological supply stores. If purchasing Didinium and Paramecium for educational purposes my experience indicates that Didinium appeared one day after receiving them and should be observed within a day or two or they may all be gone (alternatively store them in a fridge if you plan to examine them more then 4 days later). Light microscopy is an excellent method for studying these organisms behaviour. Further research is required to determine how Didinium is able to quickly identify organisms suitable to feed on after contact. It is also would be of interest to determine if Paramecium are able to detect Didinium at a distance. In some of my observations Paramecium did seem to detect the Didinium and in others (e.g. the movie) Paramecium behaved as if they did not detect Didinium and would swim between Didinium. The attachment and feeding process of Didinium occurs so quickly it is difficult to observe the steps involved with feeding. The hunt itself is interestng to watch and serveral studies show that Didinium is so efficient that in evironments where Paramecium can't hide results in all of the Paramecium being eaten within a few days. Studies on Didinium and Paramecium also show that these single-cell studies can contribute important information and reveal potential problems that may be faced in biological animal control.
Acknowledgment: I would like to thank Brandon Berdan, Mike Codner and Bruce Taylor for reading my article, suggesting changes and identifying errors.
Note: Educators and students may use my web images freely for reports and teaching, all other uses or for commercial use please contact me. If you use my images for non-profit purposes on a website or social media I appreciate attribution and a link back to this web site. I welcome inquiries and comments about microscopy or the microscopic organisms.
References & Websites
A. Banerrji et al. (2015) Density and trait-mediated effects of a parasite and predator in a tri-trophic food web. J. Animal Ecology 84: 723-733.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4674981/
J. Berger (1980) Feeding Behaviour of Didinium nasutum on Paramecium bursaria with Normal of Apochlorotic Zoochlorellae. J. General Microbiology 118:397-404. https://www.microbiologyresearch.org/content/journal/micro/10.1099/00221287-118-2-397
L. S. Luckinbill (1975) Coexistence in Laboratory populations of Paramecium aurelia and its Predator Didinium nasutum. Ecology 54:1320-1327.
https://esajournals.onlinelibrary.wiley.com/doi/abs/10.2307/1934194
H. Wessenberg and G. Antipa (1970) Capture and Ingestion of Paramecium by Didinium nasutum J. Protozool. 17. 250-257.
https://www.researchgate.net/publication/230287213_Capture_and_Ingestion_of_Paramecium_by_Didinium_nasutum
S.O. Mast (1909) The reactions of Didinium nasutum (Stein) with special reference to the Feeding habits and the function of Trichocysts.
https://www.journals.uchicago.edu/doi/abs/10.2307/1536126Feeding habits and the function of trichocysts. Biol Bulletin Vol 6 pp91-118.
Balbiani, E. G (1873) Observations sur le Didinium nasutum Arch. D. Zool. Exp. Vod 2, pp. 363-394) in S.O. Mast 1909.
T. Harumoto et al. (1998) Chemical defense by means of pigmented extrusomes in the ciliate Blepharisma japonicum. European Journal of Protistology Vol 34, Issue 4, 7 Pages 458-470
https://www.sciencedirect.com/science/article/abs/pii/S093247399880014X
J. Berger (1979) The Feeding Behavior of Didnium Nasutum on an Atypical prey Ciliate (Colpidium campylum) Trans. Amer. Micros. Soc. 98: 487-494.
https://www.jstor.org/stable/3225898?seq=1
Wikipedia Didinium - https://en.wikipedia.org/wiki/Didinium
Authors Biography & Contact Information
Bio: Robert Berdan is a professional nature photographer living in Calgary, AB specializing in nature, wildlife and science photography. Robert retired from Cell\Neurobiology research to pursue photography full time many years ago. Robert offers photo guiding and private instruction in all aspects of nature photography, Adobe Photoshop training, photomicrography and macro-photography. Portrait of Robert by Dr. Sharif Galal showing some examples of Robert's science research in the background.
Email at: rberdan@scienceandart.org
Web sites: www.canadiannaturephotographer.com
www.scienceandart.org
Phone: MST 9 am -7 pm (403) 247-2457.