
Introduction to Euglenids (Euglenoids) where some exhibit both Plant and Animal Properties
Dr. Robert Berdan
November 12, 2021

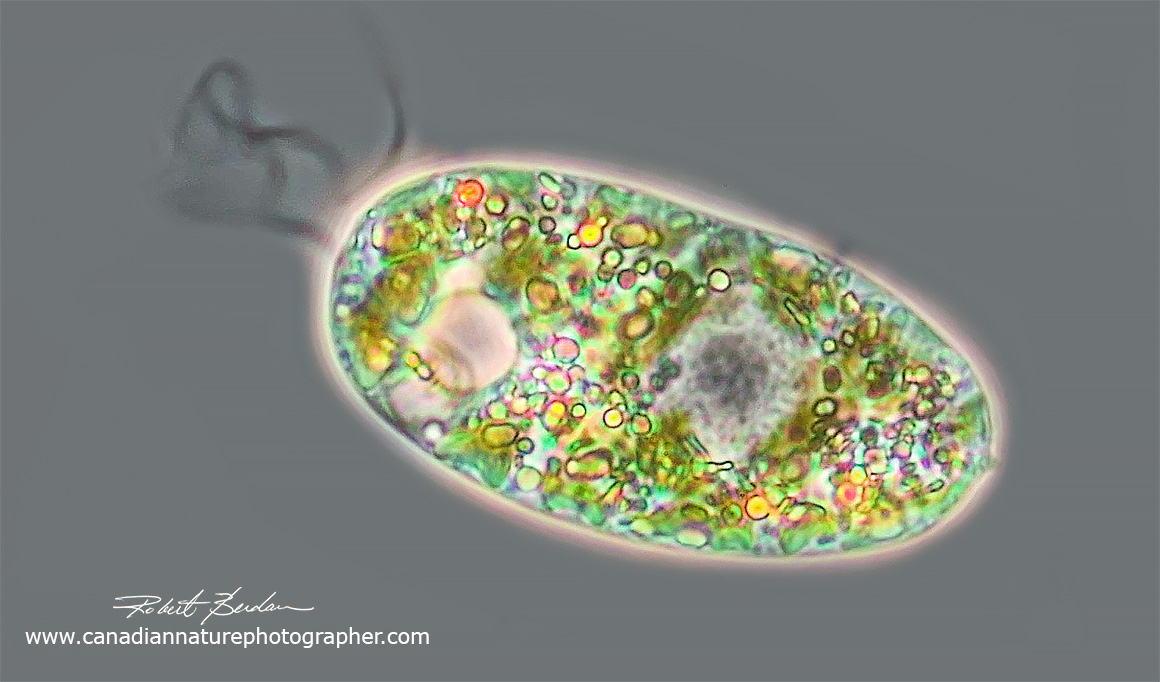
Euglenoid - Lepocinclis helicoideus
There are some tiny creatures on our planet that share both plant and animal properties e.g. Euglena that belong to the group Euglenoids. Being tiny does not mean they are insignificant. Euglenoids are found mostly in fresh water ponds, soil, ditches and they require a microscope to see them. They are single-celled, have one to four flagella (hair-like protrusions) and a pellicle made of protein strips located under their plasma membrane. Some of the euglenoids are phagotrophic and feed on bacteria or algae. Others contain chloroplasts and can produce their own food by photosynthesis or absorb nutrients from the water by osmosis – called osmotrophy. Some exhibit a mix of these nutritional characteristics (mixotrophs). Flagella permit euglenoids to swim and feed. Some have a red “eye” spot along with photoreceptors so they can respond and move toward the light. Other species can lose their chloroplasts when reared in the dark and live on bacteria or they feed on bacteria at night. These unusual organisms are used in research and may be a source of food, biofuels and pharmaceutical products in the future. Euglenoids were first observed in the 1600s by Antonie van Leeuwenhoek using his primitive single lens microscopes but anyone with a simple microscope can collect them from ponds and observe them in their home with a microscope.

A diagram of Euglena a typical Euglenoid from Wikipedia
Euglena sp. Phase contrast microscopy 400X

Euglena sp. Phase contrast microscopy 400X

Euglena sp bright field microscopy 630X. Rod shaped paramylon granules and a grey coloured nucleus is visible on the bottom right.

Euglena sp as above but viewed by darkfield microscopy. The nucleus is dark blue in this image. 630X.
Euglenozoa
The phylum Euglenozoa contains four main groups 1. Euglenids (Euglenophyceae are phototrophic euglenids) 2. Kinetoplastea (parasitic flagellates e.g. trypanosomes), 3. Diplonemids (free living having 2 short flagella of equal length and mostly marine)
4. Symbiontida (live in low oxygen marine environments and possess epibiotic bacteria).
Plant biologists classify the phototrophic euglenids as Euglenophyceae. The Euglenids (phototrophic and phagotrophic) are divided into several groups.
Eutreptiales: phototrophic flagellates mainly marine.
Euglenales: primarily freshwater with flexible or rigid pellicles made of protein strips. The group is divided into: Euglenacea, Phacaeae, Peranemacea (more groups based on molecular biology see below).
Rapaza viridis - means "green grasper" a single marine species exhibits transitional traits between phototrophic and phagotrophic euglenoids - only single species discovered by researchers in Canada (A. Yamaguchi et al., 2012).
More information about the taxonomy of Euglenids based on molecular studies is shown further below. The taxonomy of Euglenids is challenging as it depends on whether they are named by zoologists or plant biologists and identities also varies between different taxonomists (e.g. T. Cavalier-Smith, 2016). The changes in protist taxonomy due to molecular studies can make species identification beyond the abilities of citizen scientists and even some researchers.
Euglenids
Euglenids, also called euglenoids, belong to the larger group of organisms in the phylum Euglenozoa and include both free living species and parasites. Most euglenids are between 5 to 50 microns in length (micron = 0.001 mm), some can be several hundred microns or longer. In this article I show images and movies of some of these fascinating organisms and hope it might inspire readers to look at live specimens with a microscope. Most of the organisms were collected near my home in Calgary from small fresh water ponds but are found in ponds of stagnant water around the world.

Euglenoid - rigid, fusiform cell with several rod-shaped paramylon (sugar storage) grains near the posterior (pointed end on the right).
Some species e.g. Euglena sanguinea may form toxic blooms. In coastal regions some euglenids can form green patches on marine sand. Euglena gracilis is probably the most studied of the euglenids and can even be purchased online by anyone from Boreal labs to study at home or classroom all year long. Euglena gracilis is being investigated as a source of food, medicine and biofuels (A. Gissibl et al., 2019). Members of the euglenids are not known to cause disease in humans or livestock, but some may parasitize gastrotrichs and tadpoles. Some euglenozoa (e.g. Kinetoplastids) produce disease in humans (e.g. sleeping sickness, leismaniases, Chagas disease). This short article only deals with non-disease forming Euglenoids found in freshwater ponds, ditches and canals.
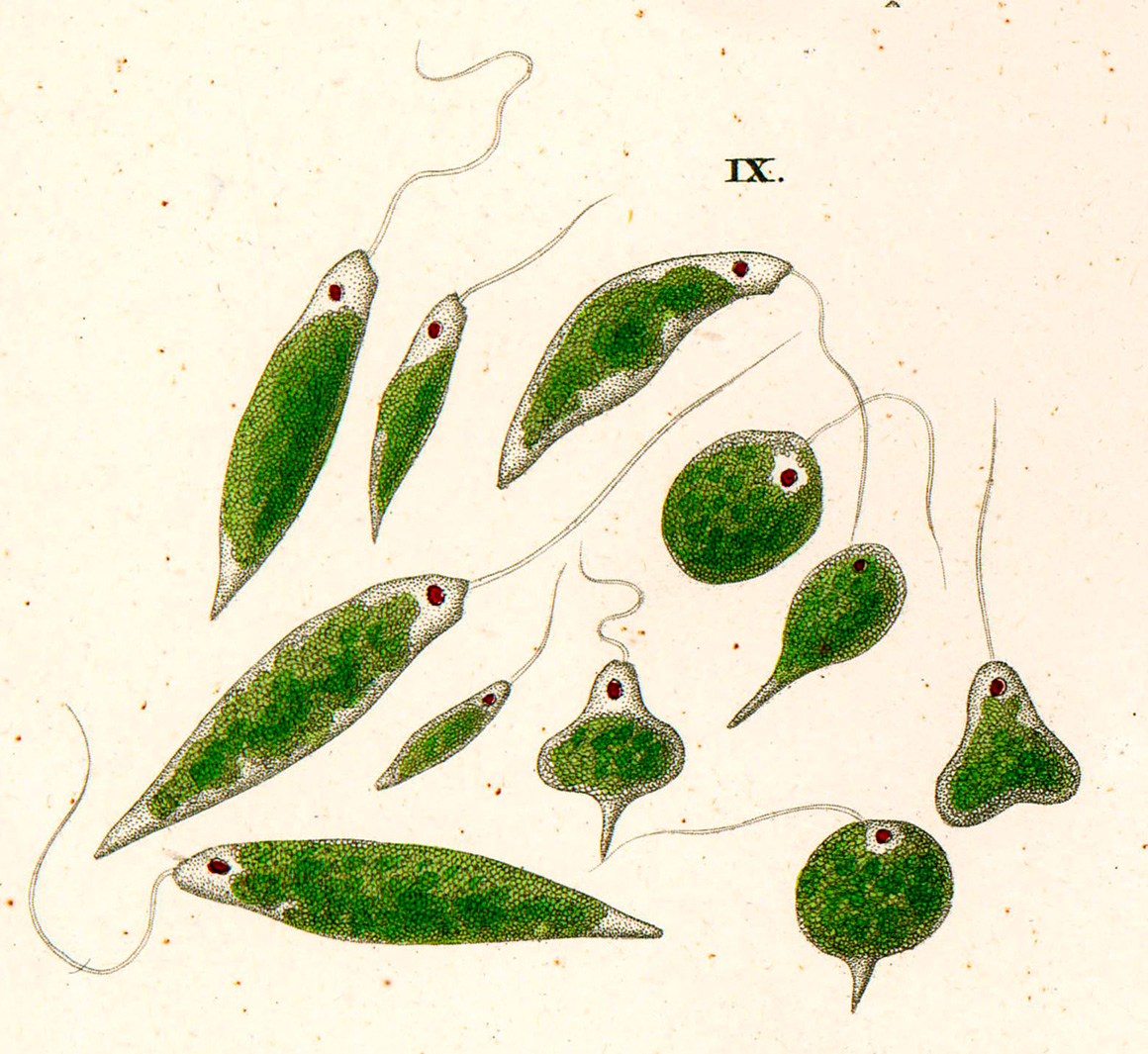
Painting of Euglena viridis, from CG Ehrenberg, Die Infustionsthierhcen, 1838 - Wikipedia
Some common characteristics of Euglenoids
Flagella originate from a pocket near the anterior and the flagella are thickened due to paraxonemal rods - one to four flagella maybe visible. Euglenoids possess a pellicle of proteinaceous strips beneath their plasma membrane that permits euglenoid movement called metaboly (G. Noselli et al., 2016) where the cell appears to thicken and narrow. This process is believed to help in the digestion of food. The main storage product of Euglenoids is paramylon a B-1,3-glucan similar to starch and this sugar polymer is being investigated for a variety of uses.

Euglena sp exhibiting movement by metaboly where the body changes shape. Image by Rogelio Moreno - Wikipedia.

Euglenoid showing long paramylon granules shaped like a stadium at the posterior end, the nucleus is ovoid near the center and a red eyespot is near the anterior end. The green coloured granules are choloroplasts. DIC microscopy 400X.
It is estimated there are about 1,500 euglenoid species (B.S. Leander et al., 2017), they have several chloroplasts of secondary origin (endosymbiosis) that are within three bounding membranes and contain chlorophylls a and b. Some euglenid species that had acquired chloroplasts earlier have become colourless (e.g. Astasia sp) and have lost their photosynthesizing ability. Euglenoids lack sexuality and reproduce by fission. Some species possess mucocysts. Mucocysts can be stained with neutral red and observed with a microscope. Mucocysts are found under the pellicle of some protists, extrusible bodies (extrusomes) of various types (e.g., trichocysts, haptocysts, toxicysts, and mucocysts) and are released from the cell usually when it is being damaged or preyed upon. Mucocysts also secrete matter used in cyst-formation, and may have other uses (B. Taylor presonal communication).

Euglenoid in a contracted shape - same euglenoid as shown in the figure above. DIC microscopy 400X. Flagellum is not visible in this photograph. The nucleus is visible as a grey oval, along with red eyespot, and numerous paramylon granules on the left side.
Benthic species of predatory euglenids and dinoflagellates are streamlined and dorsoventrally flattened and both possess extrusive organelles, or extrusomes that are similar in morphology and behaviour. Both species use flagella for transport and the recurrent flagellums sits within a groove on the ventral surface of the cell and is oriented backwards. Euglenids and dinoflagellates also possess cytoskeletal elements (paraflagellar rods within each flagellum) that are not found in any other group of eukaryotes. At different points in their evolutinary history both euglenids and dinoflagellates independently acquired photosynthesis via secondary endosymbiosis. The plastids in both groups also share 3 envelope membranes (J. Lukes et al., 2009).
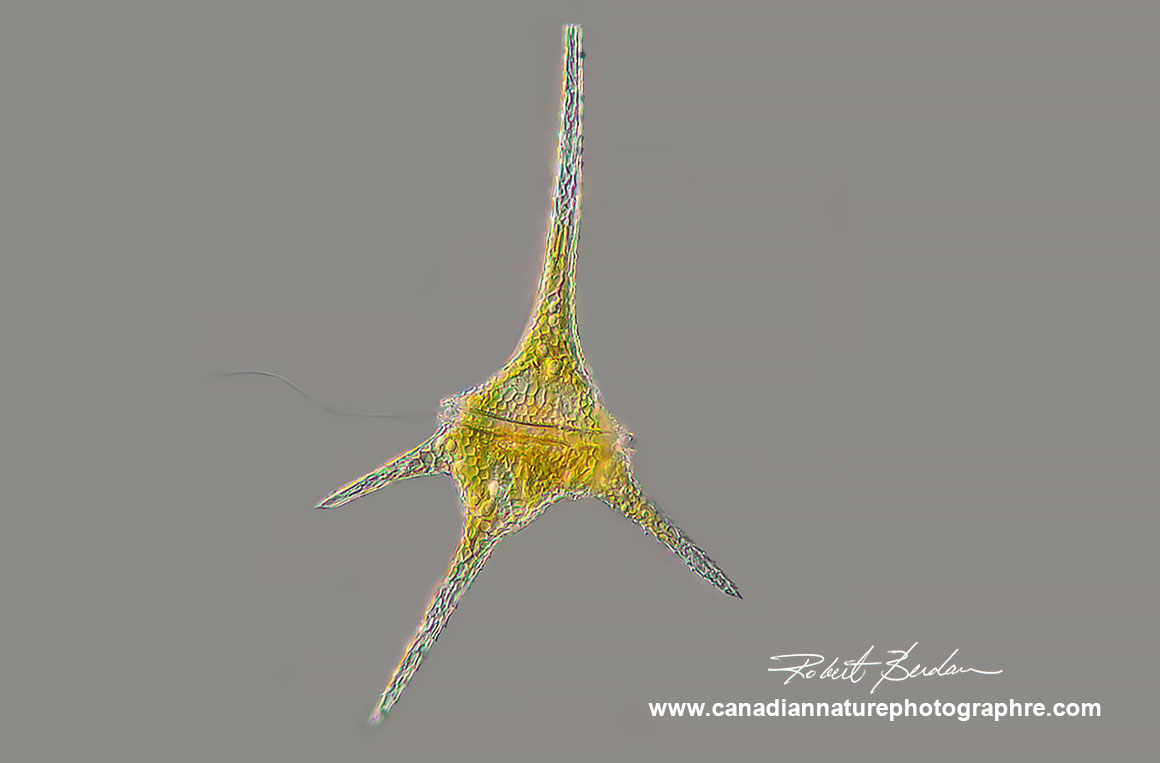
Dinoflagellate Ceratium hirundinella found in freshwater ponds and lakes. Ceratium species are characterized by their horns and two flagella located in the transverse and longitudinal positions (only one flagellum is visible in this picture). DIC microscopy 200X focus stack.
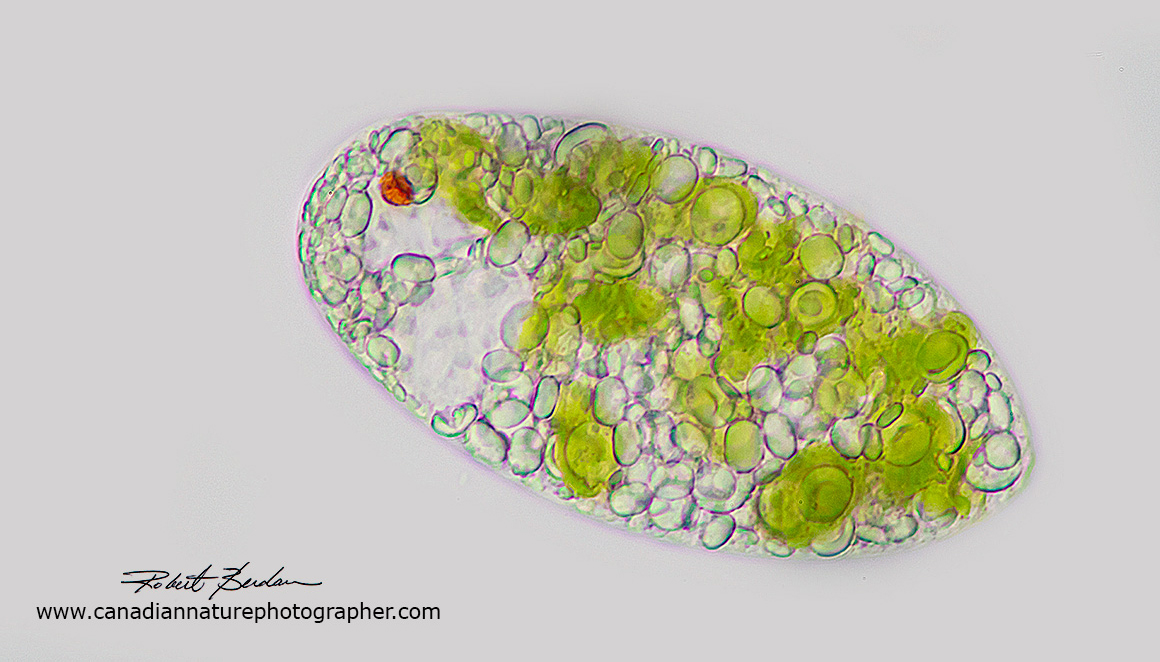
Euglenoid species by bright field microscopy. Note the red "eye" spot, translucent paramylon granules, and green chloroplasts. A flagellum is not visible in this image. 400X
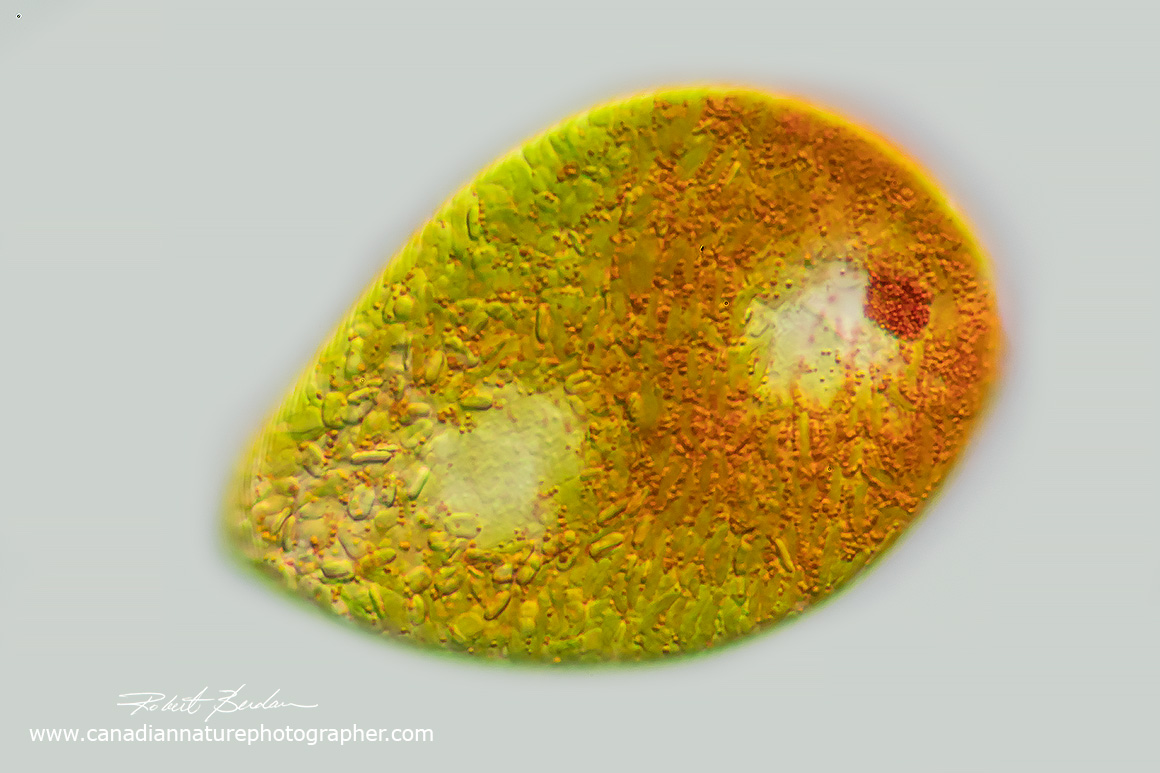
Euglenoid with red granules in the cytoplasm. Euglena sanguinea? 400X DIC microscopy.

Artwork by Allan Pentecost Wikipedia & Agriculture and Environmental Data Archive.
Euglenophyceae are divided into three groups (C. Bicudo and M. Menezes, 2016)
1. Euglenales – primarily found in fresh water, with a pellicle made up of proteinaceous strips and they swim using one emergent flagellum. This group is further divided into the Euglenacaea and Phacacea.
2. Eutreptiales are phototrophic, flexible cells with pellicle and found mainly in marine environments and have 2 or more emergent flagella.
3. Rapaza viridis – represented by a single marine species that has transitional characteristics between phagotrophic and phototrophic euglenids (Yamaguchi et al., 2012).
Euglenids have three main modes of feeding. Phagotrophy where they eat other cells like algae or bacteria, osmotrophy where they absorb nutrients from the water and phototrophy (photosynthesis). Phototrophic species respond to the direction and intensity of light using a shading stigma (red eyespot ) and photosensory swelling at the base of the emergent flagellum. Some euglenids move via metaboloy which is thought to facilitate ingestion of large food particles (Leedale, 1967). Some euglenid species have a flagellum which is thickened and possess an investment of fine hairs visible with the electron microscope. Although only one flagellum may be visible on some species, the other flagellum is often short and rests within the feeding apparatus. Phase contrast microscopy seems to be the best method for seeing the flagellum (watch the video).
It is hypothesized that endosymbiotic algae survived inside phototrophic euglenids. The green algae appears related to Pyramimonas sp. Euglenoids also possess a small pyrenoid which is a structure visible in their cytoplasm and involved in carbon fixation.
The genomes of euglenids tend to be large and include a large number of introns (noncoding sections of DNA or RNA). They also possess an unusual nucleotide base J (0.2%) in their DNA that is not found in most other organisms and this unusual base pair hampers DNA sequencing.The J base pair (hydroxymethyluracil replaces thymine) is found in some other Euglenozoa including the Kinetoplastid flagellates, Diplonema and Euglena (D. Dooijes et al., 2000).
Trachelomonas (Ehrenberg 1833)
Trachelomonas and Strombomonas are phototrophic euglenids that produce a lorica (vase-like outer coating) made of mucous that may be coloured (brown) or ornamented (K.Woloski and P.L. Walne, 2007). Strombonas separation from Trachelomonas is based on characteristics of the lorica shape, lack of a distinctive collar, possesion of a tail piece, lack of oranamention and ability to aggregate particles on the surface of the locrica. They also differ in their protein strip reduction at the anterior and posterior poles that support the separation of Trachelomonas and Strombomonas as distinct genera, though they appear to be monophyletic based on a few sequencing studies (S. Brosnan et al., 2005).
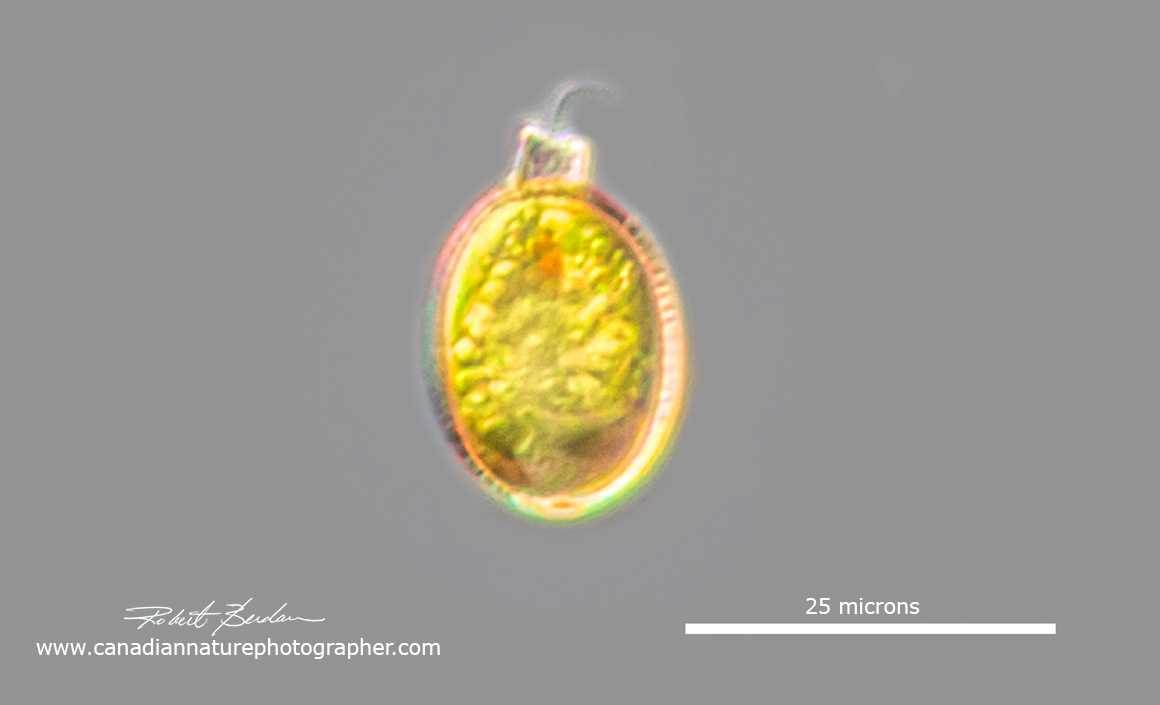
Trachelomonas sp has an eyespot and flagellar swelling, the flagella are not always visible. To notice these small euglenoids you need to be looking for them in pond samples.
Trachelomonas sp are unusual in that they live inside a lorica which is oval in shape with an apical pore through where the flagellum extends. Most if not all are found in freshwater and some occur in peaty soils with water rich in iron and manganese. Their lorica can be transparent, or brown in colour. Some are osmotrophs feeding by the absorption of nutrients by osmosis. Trachelomonas are found in North America in the littoral zone, usually warm organically polluted waters (R. Wolowski and P.D. Payne, 2007). These investigators found 5 taxa of Strombomonas and 63 of Trachelomonas.
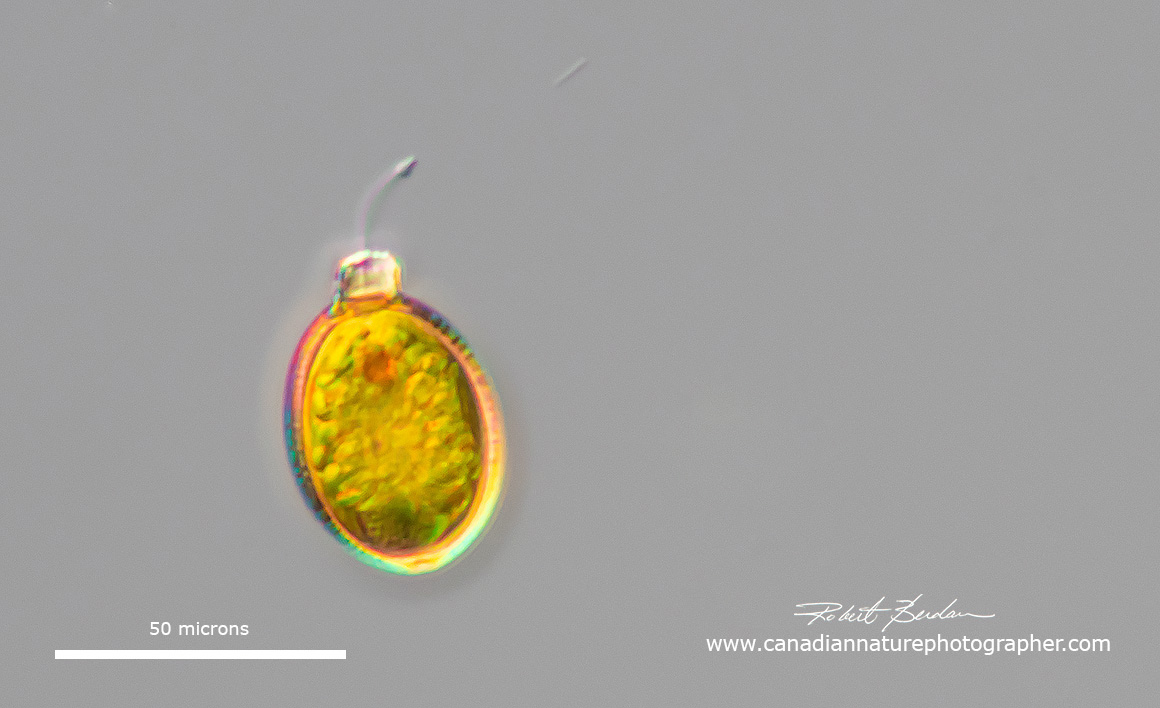
Trachelomonas sp

These two protists probably Trachelomonas sp in that they have an outer lorica-like shell, relatively small in size, an apical pore, red eye spot and a long fagellum (Trachelomonas armata?).
Colacium
One species of euglenid forms colonies and is sessile (Colacium). These euglenids are attached by branched stalks and found as ectoparasites on copepods, cladocerans and plants (A.J. Baker, 2017). I believe I have seen Colacium growing on copepods but I don't have a good picture to share at this time.
Peranema (Dujardin 1841)
Peranema has no chloroplasts and is a phagotroph feeding on euglena and other eukaryotic algae. It has two unequal lengths of flagellum, one anterior and the other is adherent to the ventral body surface and runs posterior (often not visible). The flagellum adherent to the body is difficult to see and was missed all together by early researchers ( ou can't see the posterior flagellum in my movie). The anterior flagellum is longer and thicker with a small region near the tip of the flagellum that is very active (phase contrast, DIC - Differential Interference Contrast microscopy and darkfield microscopy is helpful in seeing the flagellum). Peranema sp are about 30 to 70 microns long.
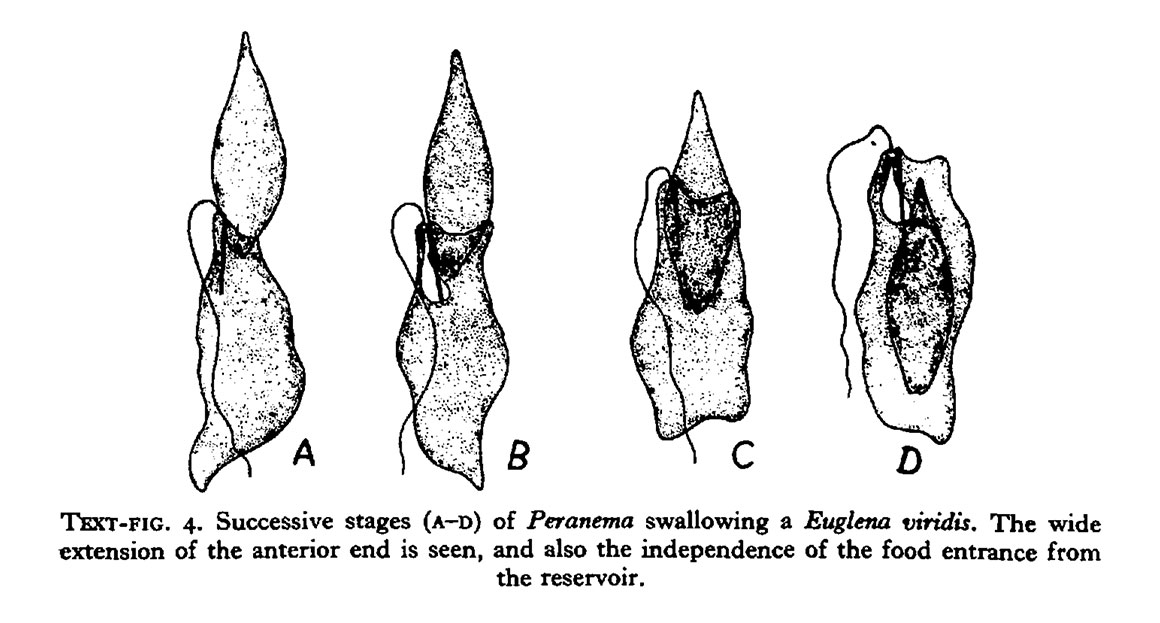
Peranema trichophorum is a phagotroph shown above feeding on Euglena viridis, diagram from (Y.T.Chen 1950). Peranema starts feeding on the anterior end of the Euglena only if the Euglena is motionless. It takes 8-15 minutes for Peranema to swallow the prey.
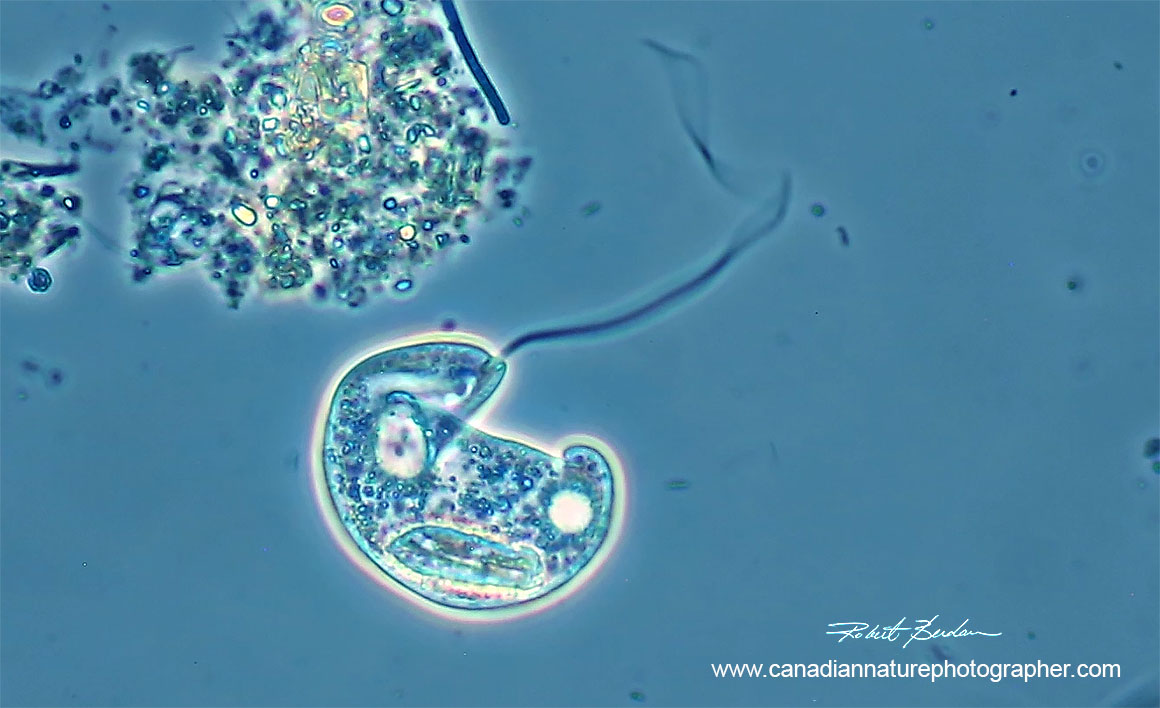
Peranema sp photographed by Phase contrast microscopy 400X. Note the long anterior flagellum.
Food preserves for Peranema are lipid and paramylon and it responds via chemotaxis to its prey. Peranema are found in stagnant ponds and the periplast when visible is finely striated. It has a contractile vacuole on its posterior end. Cannibalism occurs in Peranema and it sometimes does not swallow the entire prey but perforates it then sucks out the contents. It moves by gliding on the substratum at about 15-20 microns per second. If the flagella is cut off it grows back in about an hour (Y.T. Chen 1950).
Movie showing several live euglenids Peranema, and Euglena moving on a microscope slide.
Watch video on Youtube or full screen. Watch on Youtube - https://youtu.be/0IzR0iEma8Q
Phacus
Phacus belongs in the family of Phacacea within the Euglenaceae. These unicellular organisms often have a flat leaf-like shape with a striated pellicle, red eyespot and large central paramylon granule which is birefringent. They have a single flagellum (not always visible) and are common in freshwater. They usually live in organically enriched water bodies and are important for nutrient recycling. The organisms contain green chloroplasts but some can feed on algae and bacteria. Small crustaceans like the copepod Diaptomus and crustacean Daphnia in turn feed on Phacus sp.
There are supposedly 564 species of Phacus that have been described but only 171 species are accepted. Generally they don't posses pyrenoids (used for carbon fixation). When a flagellum is visible they generally only exhibit one, but some species have two. They possess contractile vacuoles, reproduce asexually and unlike Euglena don't exhibit movement by metaboloy. Phacus species are found in some bodies of water in large numbers and are indicators of high organic pollution.

Top are examples of Phacus acuminatus? Bottom are examples of Phacus longicauda? DIC microscopy 400X. Species identification not confirmed.
The current taxonomy of this group is based on morphology (cell size, shape, pellicle ornamentation) and molecular biology analysis (S. Kosmala et al., 2007; A. Karnkowska Ishikawa et al., 2010). Unfortunately it's not possible to carryout DNA analysis at home on protists yet, but that will change in the future (home DNA tests are available for people for paternity tests, ancestry etc by sending samples to a lab for $249). Some kits are available for DNA analysis for citizen scientists (see Bento Labs), and portable DNA sequencing is now available starting at about $1000 and can done in the field with a laptop computer (C. De. Rojas 2020).
Collecting Euglenoids

Large euglenoid

Euglenid showing red eye spot and chloroplasts 630X DIC microscopy
Generally euglenoids are not common in fast moving rivers and creeks, but tend to be more abundant in shallow ponds, ditches, canals, eutrophic and polluted waters. During the summer they can form blooms. Sometimes they are found in extreme habitats like acidic water. For collection ideally a plankton net with 10 micron pores is ideal (available on Amazon and elsewhere online). I use a coarser plankton net and also wide mouthed plastic jars. Most specimens I capture are observed with my microscopes shortly after returning home. I also ordered one batch of Euglena gracilis from Boreal labs and the Euglena survived more than nine months with only light, some deionized water and a few rice grains which I added to promote the growth of bacteria. Other euglenids can even grow on tree bark, and snow (R.W.Hoham and D. Remias, 2020). I will try collecting some algae in the Rocky mountains that are growing on snow in spring of next year.

Euglena gracilis from Boreal labs Differential Interference Contrast microscopy (DIC) 630X.

Euglena gracilis flattened by coverslip 630X DIC microscopy.
Reasons why some Euglenoids are important?
Exhibit both plant and animal properties.
They produce oxygen we breath.
Some euglenoids produce blooms with toxins.
Euglenoids are a potential food source for both humans and livestock.
Euglenoids sequester heavy metals and maybe useful in the bioremediation of polluted water.
Some species survive acidic conditions and ionizing radiation.
Paramylon could be an alternative to petroleum based resins and pressure sensitive adhesives.
Paramylon is used in drug delivery and has immunostimulatory properties.
Euglenoids might be used to produce bioethanol (currently they are not competitive with fossil fuels).

Euglena gracils
Euglena gracilis is a good source of dietary protein, lipids, vitamins (A, C and E) and paramylon (starch like storage product). Paramylon is being marketed as a immunostimulatory agent and yields are relatively high compared to other algae. Paramylon also lowers cholesterol and has antibacterial and antidiabetic activities. Paramylon has been used to treat colorectal and gastric cancers (Gissible et al., 2019). Euglena are a rich source of Vitamin A compounds (retinol, retinal, retinoic acids, retinyl esters) which act as antioxidants. Vitamin A is found only in animal products such as fish and dairy. Euglena also produces Vitamin C (ascorbate) essential for human health. Microalgae have been proposed as a source of feedstock and the production of biofuels because of high amounts of lipids they contain. Lipids can also be used as a lubricant, or raw as material for candles and cosmetics. The main challenges are scaling up the processes for industrial use and future development. For an overview of the potential benefits of E. gracilis as food, medicine and biofuels see (Gissible et al., 2019).
Examining Euglenoids
A $100 light microscope can be used to see and photograph euglenoids using a cell phone camera. I examine them with various microscopes using bright field, darkfield, phase contrast and differential interference microscope (DIC). Some researchers prefer to use Anoptral contrast microscopy (Leedale, 1967). Euglenoids can be preserved in 2% buffered glutaraldehyde or Bouin's fixative (available from Amazon) when they can't be examined shortly after collection.

Lepocinclis fusca (originally Euglena fusca) has triangular shaped ornamentation (papillae) on the pellicle (Kosmala et al. 2005). This species was renamed following molecular biology studies. Two large paramylon granules are visible inside the cell along with a red eye spot. 400X DIC microscope.
Photography
Euglenoids are challenging to photograph. You need a light microscope having 100 to 400X because euglenoids tend to be small, they often twist during locomotion and the thickness of the overlying coverslip is important to get good photos. One way I try to improve my photomicrography of these organisms is to use No. 1.5 coverslips from Zeiss (0.170 mm +\- 0.001 mm) and flatten the coverslip on top of the euglenoids by extracting excess water below the coverslip using a piece of paper towel. Alternatively I wait until the water evaporates from under the coverslip for about an hour. The movement of euglenoids is not particularly fast, but they twist and turn and turn and change shape (metaboly). I sometimes use focus-stacking to increase the depth of field of the images captured. For more specific information on how to photograph specimens with a microscope see my articles “Tips on How to take better pictures with a Microscope – photomicrography” and " Cell Phone Cameras, Dedicated Digital Cameras and Digital Single Lens Reflex Cameras for Photomicrography". Taking pictures with a microscope and cell phone camera is an easy way to begin to record your observations with a microscope.
If you are interested in observing euglenoids visit a local pond or purchase some live specimens from a biological supply house (Boreal labs). You will need a good introductory microscope (not a toy one) – they can be found used on Kijjii starting around $100. The microscope should have a bright light source with at least 10, 20 and 40X objectives (4X and 63X or 100X objectives are also nice to have).

Lepocinclis fusca

Above two photomicrographs (top) bright field microscopy (bottom) DIC microscopy 400X. Images show the flexibility of this large euglenoid and the pellicle ornamented with triangular shaped structures on the pellicle. Lepocinclis fusca (previously Euglena fusca). L. spirogyroides is similar in morphology but has cuboid shaped papillae.
Taxonomy of Euglenids - overview
Domain: Eukaryota
Kingdom: Protista (neither plant or animals)
Phylum:
Euglenozoa (Cavlier -Smith, 1981) ICZN
Euglenophyta (Pascher, 1931) ICN
ICZN - International Commission on Zoological Nomenclature
ICN - International Code of Nomenclature for algae, fungi, and plants
Classes:
Diplonemas - mostly marine, deep sea, benthos - heterotrophic ~ 45,000 species
Euglenoidea\Euglenophyceaen - Symbiontida and Euglenida
Symbiontida - marine heterotrophs, anoxic marine, sulfide epibionts
Euglenida -paramylon, phototrophic, osmotrophic, mixotrophic
Kinetoplasta - many parasitic species - Trypanosomids
Order: Euglenales - animal classification
Euglenophyta - plant classification
Family: Euglenophyceae (Photoautotrphic euglenids)
Eutreptiales - marine genera Eutreptia and Eureptiella
Phacaceaea - Discoplastis, Lepocinclis, Phacus
Euglenacaea - Euglena, Monomorphina, Cryptoglena, Euglenaria, Euglena acrchaeoplastidiata, Trachelomonas Strombonas, Coalcium, Euglenaformis
Genus: Euglena, Phacus, Rapaza. Lepocinclis etc
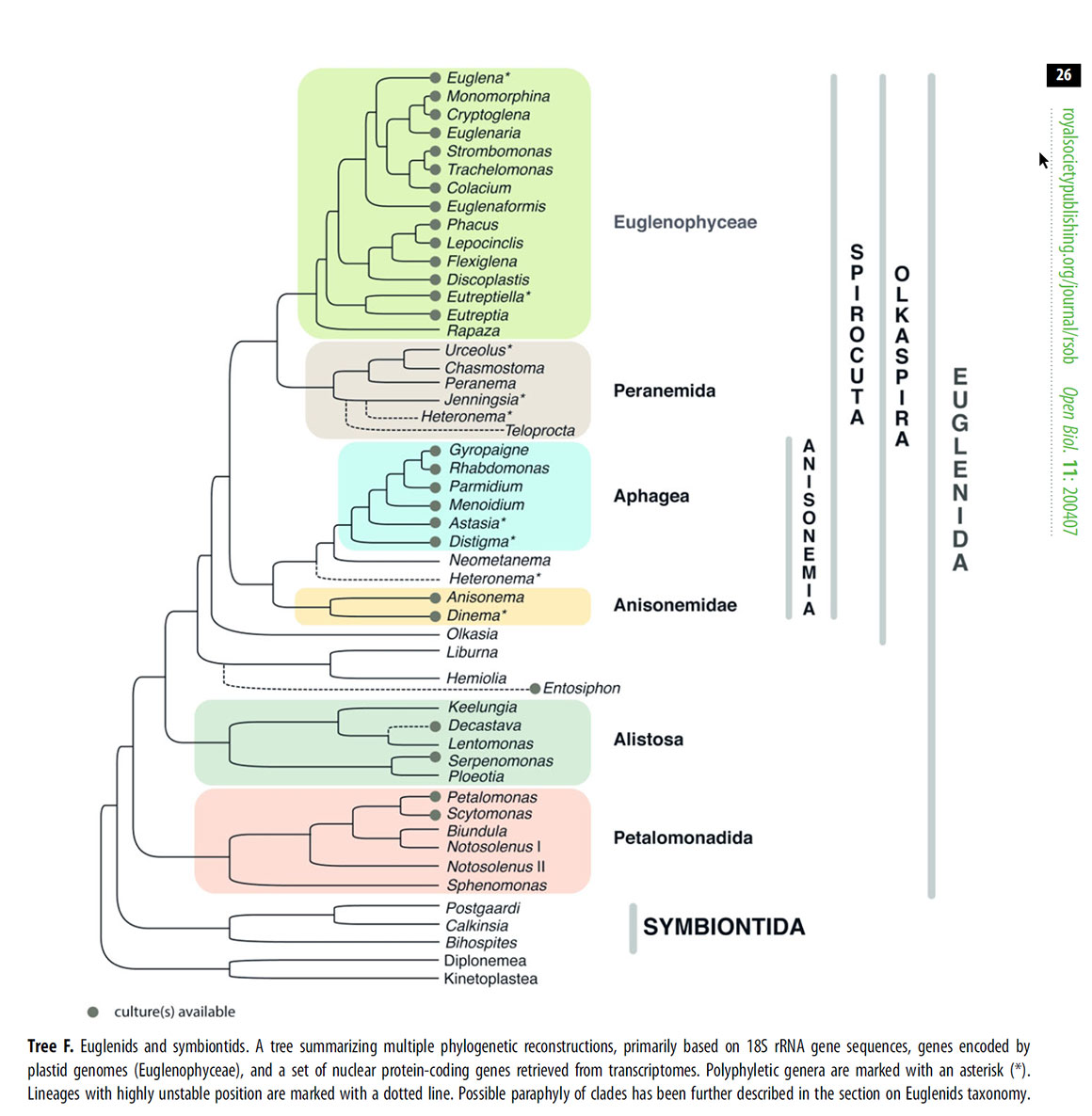
Above open source diagram from https://royalsocietypublishing.org/doi/10.1098/rsob.200407 (A.Y. Kostygov et al., 2021) showing phylogenetic construction of Euglenids and Symbiontids based on 18S rRNA gene sequences).
Summary
Euglenoids belong to the Phylum Euglenozoa (Euglenophyta) and sone share both plant and animal properties (chloroplasts, movement). Some euglenoid species exhibit photosynthesis, other feed on other algal cells and bacteria and few are able to absorb nutrients via osmosis. Their protein pellicle, and flagellum are shared characteristics. Some euglenids can produce toxic blooms. Euglenids are important in the food web, provide oxygen and are being considered as a potential food source and for some pharmaceutical agents. Euglenoids are common in fresh water ponds and can be purchased online for study at home or the classroom with a microscope. Most fresh water ponds or stagnant bodies of water will have some euglenids and new research may reveal their full potential for use in medicine, food or as a source of fuel.
Acknowledgements: I thank Bruce Taylor for species confirmations, taxonomic information and typographic corrections. Bert van Helden for drawing attention to an error involving Dinoflagellates. My online photos are available free for educational and non-commercial use (please include link and credit). Larger images for printing or commercial use can purchased by contacting me. I offer microscope training, and pond collecting outings to anyone interested.
References
A.Y. Kostygov, A. Karnkowska, J. Votypka, D. Fashyreva, K. Maciszewski, V.Yurchenko and J. Lukes (2021) Euglenozoa: taxonomy, diversity and ecology, symbioses and viruses. Open Biol. 11: 200407 https://royalsocietypublishing.org/doi/full/10.1098/rsob.200407
Euglenids (2021)
A.Gissible, A. Sun. A.Care, H. nevalainen and S. Sunna (2019) Bioproducts from Euglena gracili: Synthesis and Applications. Frontiers in Bioengineering and Biotechnology. 7:1-16.
https://www.frontiersin.org/articles/10.3389/fbioe.2019.00108/full
Euglena Wikipedia (2021) Overview, pictures, video and references
C. De Rojas (2020) Portable Sequencing is Reshaping Genetics Research - Labiotech.au
https://www.labiotech.eu/in-depth/portable-sequencing-genetics-research/
R.W. Hoham and D. Remias (2020) Snow and Glacial Algae: A review. J. Pycol. 56:264-282.
https://onlinelibrary.wiley.com/doi/10.1111/jpy.12952
B.S. Leander, G. Lax, A. Karknowska and A. G.B Simpson (2017) Euglenida in Handbook of Protists, Springer International. Pp 1-41.
http://www3.botany.ubc.ca/bleander/images/EuglenidaHandbook.pdf
A.J. Baker (2017) Euglenoids (Euglenophyceae) an Image Based Key (Phyco Key). University of New Hampshire. http://cfb.unh.edu/phycokey/Choices/Euglenophyceae/Eugleno_key.html
C. E. de M. Bicudo and M. Menezes (2016) Phylogeny and Classification of Euglenophyceae: A Brief Review. Frontiers in Ecology and Evolution. 4: 1-15.
https://www.frontiersin.org/articles/10.3389/fevo.2016.00017/full
G. Noselli, M. Arroyo, A. Beran and A. DeSimone (2016) Metaboly in Euglenids: A Model and its Experimental Validation. XXXIV ICTAM 21-26. Montreal, Canada.
J. J. Kim, E.W. Linton and W. Shin (2016) Morphological and genetic diversity of Euglena deses group (Euglenophycaea) with emphasis on cryptic species. Algae 31: 219-230.
https://www.e-algae.org/journal/view.php?number=2786
T. Cavalier-Smith (2016) Higher classification and phylogeny of Euglenozoa. Europ. J. Protistology. 56:250-276.
https://www.sciencedirect.com/science/article/pii/S0932473916300839
A.Yamguchi, N. Ubuki and B.S. Leander (2012) Morophostasis in a novel eukaryote illuminates the evolutionary transition from phagtrophy to phototrophy: description of Rapaza viridis n. gen. et. https://bmcecolevol.biomedcentral.com/articles/10.1186/1471-2148-12-29sp (Euglenozoa, Euglenida). BMC Evolutionary Biology: 12: 29-45.
V. P. Edgcomb, S. A. Breglia, N. Yubuki, D. Beaudoin, B.S. Leander and J.M.Bernhard (2011) Identification of epibiotic bacteria on symbiontid euglenozoans in O2 depleted marine environments: evidence for symbiont and host co-evolutions. The ISME Journal 5: 231-243.
https://www.nature.com/articles/ismej2010121
A. Karnkowska-Ishikawa, R. Milanowski, J. Kwiatowski, and B. Zakrys (2010) Taxomy of the Phascus oscillans (Euglenaceae) and its close relatives balancing morphological and molecular features. J. Phycol. 46: 172-182. https://www.researchgate.net/publication/240654466_Taxonomy_of_Phacus_oscillans_
Euglenaceae_and_its_close_relatives-balancing_morphological_and_molecular_features
J. Lukes, B. S. Leander and P. J. Keeling (2009) Cascades of convergent evolution: The corresponding evolutionay histories of euglenozoans and dinoflagellates. PNAS 106:9963-9970.
http://www3.botany.ubc.ca/bleander/images/PNASEugDino.pdf
S. Kosmala, M. Bereza, R. Milanowski, J. Kwiatowski, and B. Zakrys (2007) Moprhological and molecular examination of relationships and epitype establishment of Phacus pleuronectes, Phacus orbicularis, and Phacus haeelii. J. Phycol. 43:1071-1082.
Research Gate - PDF
K. Wolowski and P.L. Walne (2007). Strombomonas and Trachelomonas species (Euglenophyta) from south-eastern USA. European J. Phycology. 42: 409-431.
https://www.tandfonline.com/doi/full/10.1080/09670260701702508
S. Brosnan, P.J. P. Brown, M.A. Farmer and R.E. Triemer (2005). Morphological separation of the Euglenoid Genera Trachelomonas and Strombonas (Euglenophytae) based on Lorica development and posterior strip reduction. J. Phycology 41: 590-605. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1529-8817.2005.00068.x
B. S. Leander, R.E. Triemer and M.A. Farmer (2001) Character evolution in heterotrophic euglenids. Europ. J. Protistol. 37: 337-356.
https://www.sciencedirect.com/science/article/abs/pii/S0932473904700280
D. Dooijes, I. Chaves, R. Kieft, A. Birks-Mulder, W. Martin and P. Borst (2000) Base J oringinally found in Kinetoplastida is also a minor constituent of nuclear DNA of Euglena gracilis. Nucleic Acids Research 28: 3017-3021. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC108458/
Y.T. Chen (1950) Investigations of the Biology of Peranema trichophorum (Euglenineae). Quarterly Journal of Microscopical Science 91:279-308. - Download PDF
Bibliography
R.E.Triemer and B. Zaktys (2015) Photosynthetic Euglenoids In Freshwater Algae of North America - Ecology and Classification ed. by J.D. Wehr, R. G. Sheath and J.P. Kociolek Chapt 10 pp 459-484. Academic Press. Toronto.
D.E. Buetow ed (1968) The Biology of Euglena Vol II Biochemistry. Academic Press New York. Pp 1-407.
G.F. Leedale (1967) Euglenoid Flagellates. Prentice Hall Biological Sciences Series Englewood Cliffs, N.J P1-223. Includes a key, excellent drawings and photomicrographs.
Authors Biography & Contact Information
Bio: Robert Berdan is a professional nature photographer living in Calgary, AB specializing in nature, wildlife and science photography. Robert retired from Cell\Neurobiology research to pursue photography full time many years ago. Robert offers photo guiding and private instruction in all aspects of nature photography, Adobe Photoshop training, photomicrography and macro-photography. Portrait of Robert by Dr. Sharif Galal showing some examples of Robert's science research in the background.
Email at: rberdan@scienceandart.org
Web sites: www.canadiannaturephotographer.com
www.scienceandart.org
Phone: MST 9 am -7 pm (403) 247-2457.