
Photography of Chaoborus the phantom midge fly larva - Zooplankton's Nightmare
Macrophotography, and photography with a Stereomicroscope and Light microscope
by Dr. Robert Berdan
May 27, 2020

Chaoborus larva head photographed with polarized light microscopy 200X
Introduction
There are aliens on earth, not the ones you hear about on the History channel, but aliens that look stranger than you might imagine. Some of them live in the deep sea, but you can also find them in nearby lakes and ponds and discover them with a microscope. As soon as the ice opened up over small ponds in the Calgary area I was out collecting aquatic invertebrates (beginning of April). I was hunting for diving beetles, dragon fly larvae and anything else I might photograph. On the second weekend out collecting I scoured the edges of the ponds and when I got back to my home laboratory I found several Chaoborus larvae for the first time. I had seen pictures of them before in books, but I had never collected any live specimens before.
There are two reasons I might have missed them in the past. The first is that they are transparent and because of this feature are called Glassworms. Also most of my collecting is done in the summer months and I found Glassworms on the second weekend in April. Most of the ponds up to that time were still covered in ice as we had a long winter this year. In the water they resemble mosquito larva and are several millimetres long and wiggle about. Up close when viewed with a microscope they have predatory dinosaur-like heads. In fact they are fierce predators that prey on plankton like: copepods, cladocerans, mosquito larva, rotifers and even ciliates.

Life cycle of Chaoborus illustrating the various stages (eggs,larvae, pupae, adult). Diagram courtesy T.U. Berendonk et. al. 2009 - PDF.
The larvae belong to a group of midge flies, there are 44 species that are found in fresh water lakes and ponds all around the globe. The female lays about 200-300 eggs in rafts that survive over winter as do some adult 4th instar larvae. The rate of their development is temperature dependent. In spring the 4th instar adults form a pupa which can emerge after 2-4 days when the water is 20 °C, 10-13 days at 10 °C or 30 days at 5 °C. An adult non-biting fly emerges and lives only 1-10 days. They don't feed at this time, though they can form large swarms. In some countries residents collect and eat the flies.

Above Figure A – male adult Chaoborus astictopusa; B – pupa C. trivittatus; C – fourth instar larva C. trivittatus; D – wing of C. americanus; E – paramere (part of the external reproductive organs of male insects) of C. flavidulus; F–G paramere of C. annandalei from two different perspectives. (Sources: A, Herms 1937; B–C, Borkent 1979; D, Cook 1981; E–G, Edwards 1930) - diagram from Art Borkent (2012) Research Gate.
The larval form of Chaoborus (pronounced k-o-borus) is eaten by fish and those that like to fly-fish sometimes make artificial lures to try and catch fish. Making Chaoborus lures is not an easy task as they are transparent (see the web site flyguys.net). The larva are similar in appearance to mosquito larva (see below), they move erratically and unless you are looking for them they might not be noticed. I caught a dozen larvae in a shallow pond in Calgary northwest while searching for diving beetles and dragon fly larvae. When I viewed Chaoborus larvae with a stereo and light microscope I was amazed at what I saw.
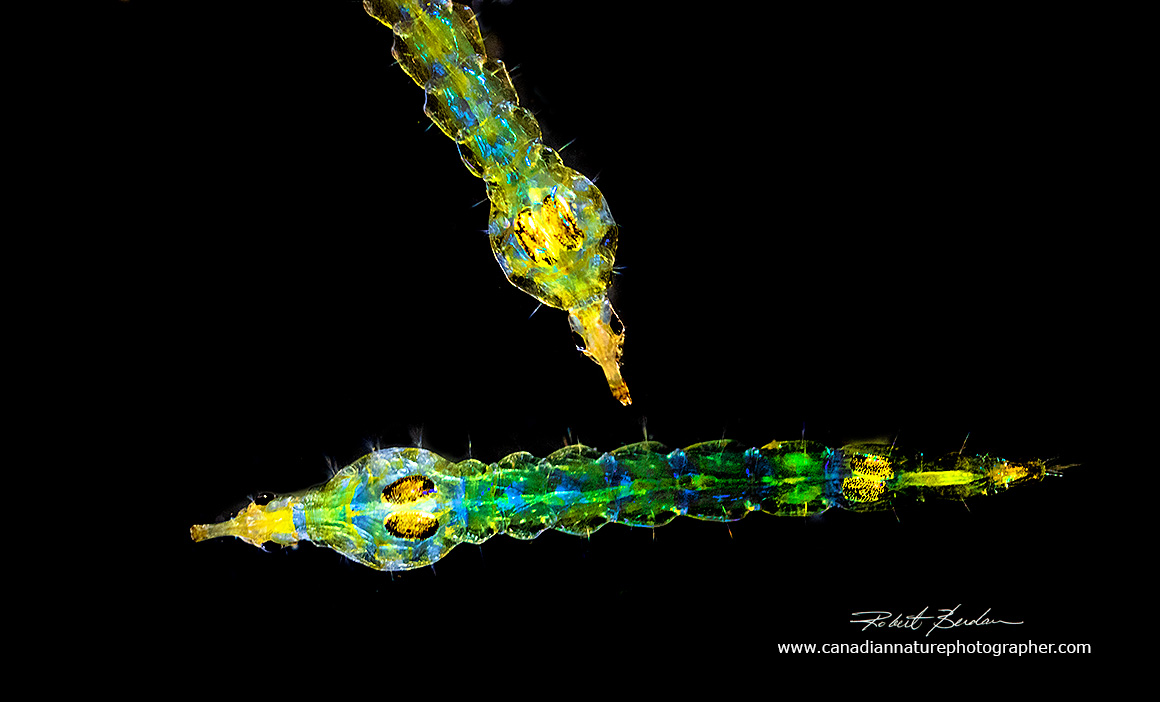
Above is a pair of Chaoborus larva photographed with a Zeiss Stemi stereomicroscope looking down on the specimens. I placed the larva in a shallow dish of water and used a combination of fiber optic lighting and a ring-flash. The camera used was a Nikon 7500 attached to the Stemi microscope port (see below). 10X

Above - this pair of Chaoborus larva were photographed in a mini-aquarium made of two sheets of glass (about 5 x 7 inches) separated by about 4 mm (1\4 inch) piece of water-proof sheet of foam from the hardware store. I clamped the two sheets together to form a water proof seal and added two metal L brackets so the glass would stand upright (see photos below). I cut a slot in the top of the foam to make a small enclosure about 1 inch long (24 mm) and 1\2 inch high (13 mm) and 1\8 inch deep (3 mm). The most obvious Chaoborus features are their tail fans and heads with large compound eyes and prehensile antenna designed to catch prey (looks like a hook). I created a black background by placing black paper behind the mini-aquarium and the light reflected from the flash coloured the muscles. 10X
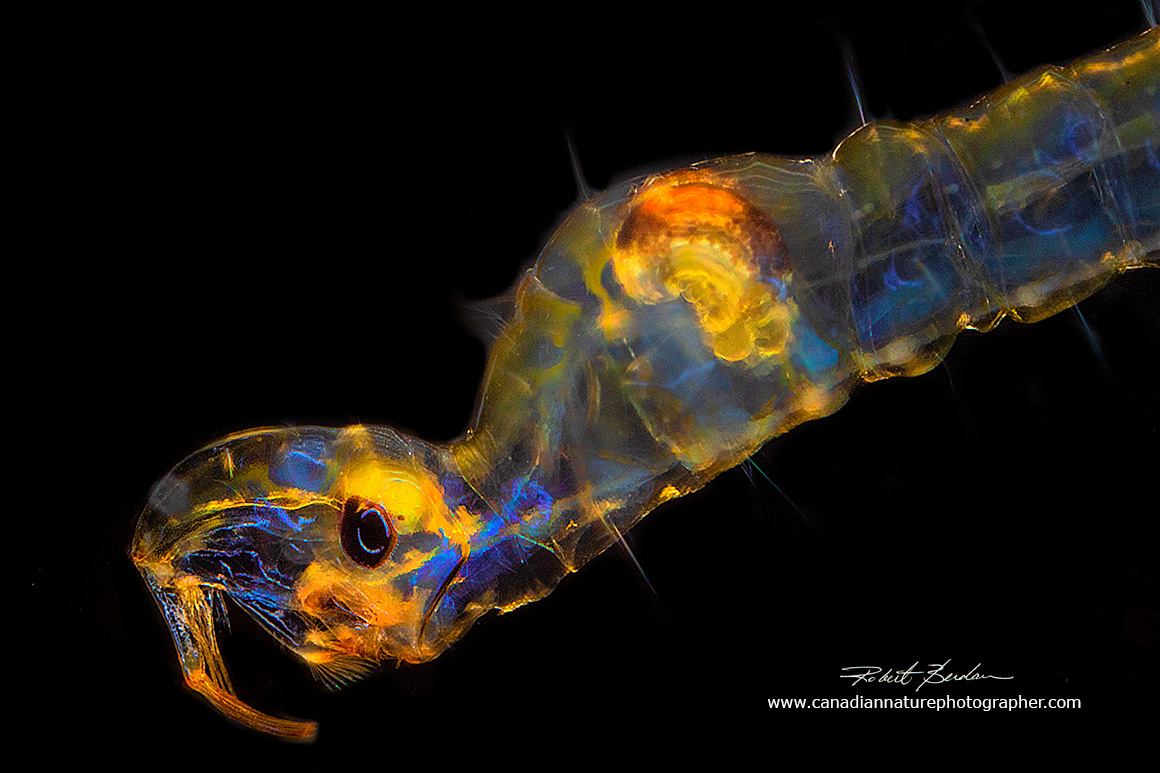
Chaoborus larva photographed with a Canon MP-E 65 mm macro lens and Canon ring flash in the mini-aquarium I made (shown below). About 20X

Above - Chaoborus larva floating just below the water surface in the mini-aquarium 20X

The tail fan was made up of 24 filaments which facilitates the extremely fast movements of Chaoborus. I combined darkfield and polarized microscopy for this picture. 20X

Live Chaoborus viewed by DIC (Differential interference microscopy). Note the 2 pairs of C shaped air sacs that control the organisms buoyancy.

When Chaoborus larva are viewed with polarized light and a full wave retardation plate (525 nm) the birefringent colours of the muscles are enhanced. If the polarizing filters are placed 90 degrees to each other (called the extinction position) the background is black, but if they are placed at some intermediate angle to each other you can produce a neutral gray background.

Above is the head of Chaoborus larva photographed with DIC microscopy (100X).

Overview of Chaoborus obscuripes larval head morphology. left: microscopic image, right: micro-CT based reconstruction. ant = antenna basis; ase = antenna's setae; cey = compound eye; lab = labral setae; mabe = musculus abductor epipharyngis; mabm = musculus abductor mandibulae; made = musculus adductor epipharyngis; madm = musculus adductor mandibulae; maf = mandibular fan; man = mandible; max = maxilla; mra = musculus retractor antennae; pre = prelabral appendages; rhi = rhinopharynx; ros = rostrum. Scale bar = 500 μm. One micron = 0.001 mm From https://doi.org/10.1371/journal.pone.0214013.g001 (Kruppert et. al. 2019).
The nightmarish attack of a phantom midge larva - you can also watch this video on YouTube (source S. Kruppert et. al. 2019- full reference below). Note that the Daphnia does not seem to always touch the Glassworm though it might trigger some sensory bristles not seen. This raises the question of whether Chaoborus hunts by sight or may detect prey by its movement or vibrations in the water caused by the preys movement?

Chaoborus larvae produced by stitching several images together. The images were taken with a Polarizing light microscope and full wave 550 nm retardation plate which enhances the birefringence and adds colour to the background. The photo above shows two pairs of black air sacs and the numerous segmental muscles. The colour of the muscles depends on their orientation in polarized light 20X.

Above is Chaoborus with a pair of C shaped air sacs in the anterior region photographed with polarized light microscopy. The air sacs allow Chaoborus to control its buoyancy. The individual muscles are birefringent and can be clearly seen through the transparent cuticle. 100X

Pair of posterior air sacs in Chaoborus sp. 200X using polarization microscopy. The larvae can adjust the volume of air in these sacs to control their buoyancy by changes in the sac walls allowing Chaoborus to rise, dive or float horizontally at particular levels within the water column. In lakes Chaoborus often buries itself in the mud during the day, then floats to the surface to feed at night.
Chaoborus is the only insect known to control buoyancy by regulating the volume of internal gas-filled sacs. There is no musculature that could be acting on the walls of these semi-rigid sacs and the mechanism by which they do this is unknown. One preliminary study suggests that the contracting force is generated by the sac wall in response to changes in pH and ionic strength (E. McKenzie and P.G.D Matthews, 2018).

Chaoborus larva viewed by Fluorescence microscopy. The specimen was bathed in Acridine orange (1µg\ml) for 20 minutes and photographed with a 4X objective using blue light excitation with a Nikon Epifluorescence microscope. 100X

Chaoborus larva photographed between crossed polarizers without a retardation filter. Most of the muscles are bright and a few are coloured. The addition of a retardation filter creates colour in the muscle by light interference. This is a panorama made by stitching several images together. 20X

Chaoborus larva in polarized light with the polarizing filters not in extinction and with a full wave retardation filter. 40X

Chaoborus larva viewed by bright field microscopy. The dark air sacs and large compound eye are conspicuous. Internal muscles can be seen but not as clearly as with polarized light microscopy. 40X

Chaoborus larva in which several images were stitched together to show the entire organism. The gut can be seen clearly in this image as a dark brown tube. Brightfield light microscopy. 20X

Head of Chaoborus photographed by Phase contrast microscopy. 100X. Phase contrast is a microscopy technique that causes materials of different thickness and refractive index to become darker or lighter, but this technique does not add colour.

Two pictures of the head of Chaoborus Left: Brightfield microscopy Right: Polarized light microscopy. This specimen had long flaps under its chin which was unusual.

When I examined Chaoborus at high magnification with my microscope using a combination of darkfield and polarization microscopy ( a very strong light source to do this), I found Bdelloid rotifers live on the insects cuticle at 400X. I watched the rotifers extend and contract as they filtered bacteria and other small organisms from the water. It is common to find protists of various types living on other larger aquatic micro-organisms. Chaoborus feeds on rotifers, but living on the animals back is one way to avoid predation. Below is a short video of the rotifers living on Chaoborus.
Movie of Bdelloid rotifers living on Chaoborus larva - alternatively you can view movie on YouTube.
Pupa
When the Chaoborus larva reaches its 4th instar it soon forms a pupa where it undergoes metamorphosis into an adult fly within a few days depending on the temperature of the water. The pupa has well developed terminal paddles and large compound eyes. The adult wings can be seen forming through the cuticle below the "chin". The pupa does not feed, but it can float near the surface or sink and it continues to "wriggle" making macrophotography challenging. The structures at the top resembling "rabbit ears" are respiratory organs.
Also see several high magnification photomicrographs of Chaoborus pupa by Jacek Myslowski (2018) on the Canadian Nature photographer web site.

Pupa floating under the surface in a mini-aquarium. Photographed with a Canon MKII camera, Canon ring flash and the Canon MP-E 65 mm macro lens. Bubbles, debris and spots on the glass were removed using Photoshop.

Chaoborus larva hanging vertically in a mini-aquarium. The ear-like structures at the top of the head are respiratory organs. The pupa do not feed. 30X

Composite image of Chaoborus larvae photographed in a mini-aquarium. 20X

Aliens face off - Chaoborus pupa - composite image 20X

Side view of Chaoborus pupa by bright field microscopy and frontal view by dark field microscopy.20X These larvae exhibit some resemblance to the creatures in the movie "Aliens".
Food for Chaoborus
Chaoborus feed on copepods, Cladocerans (e.g. waterfleas), rotifers and even large ciliates. Their favourite foods seems to be copepods that can swim up to 102 mm per second, while Daphnia's swimming speed is only about 15 mm per second. Chaoborus is an ambush hunter as it sits and waits until an organism touches one of its sensitive hairs that in turn triggers a feeding response shown above in the movie.

Copepods are small crustaceans common in both salt and fresh water. They can swim quickly which you can discover for yourself by trying to catch one with an eye dropper. They are 0.5 to 2 mm in length. Note the single red eye on the anterior end which is sensitive to light and movement. At the posterior end are two egg sacs. Photographed with Polarized light microscopy 100X in order to show the birefringent muscles. Daphnia respond to kairomones that Chaoborus releases into the water and the Daphnia grow "neck-teeth" on its dorsal surface which seems to have a protective effect against predation. These spines are quite small and its difficult to imagine how they could have much of an effect, but several research papers indicate they do (Juracka et. al.2011 and Dennis et. al. 2014).
Water fleas
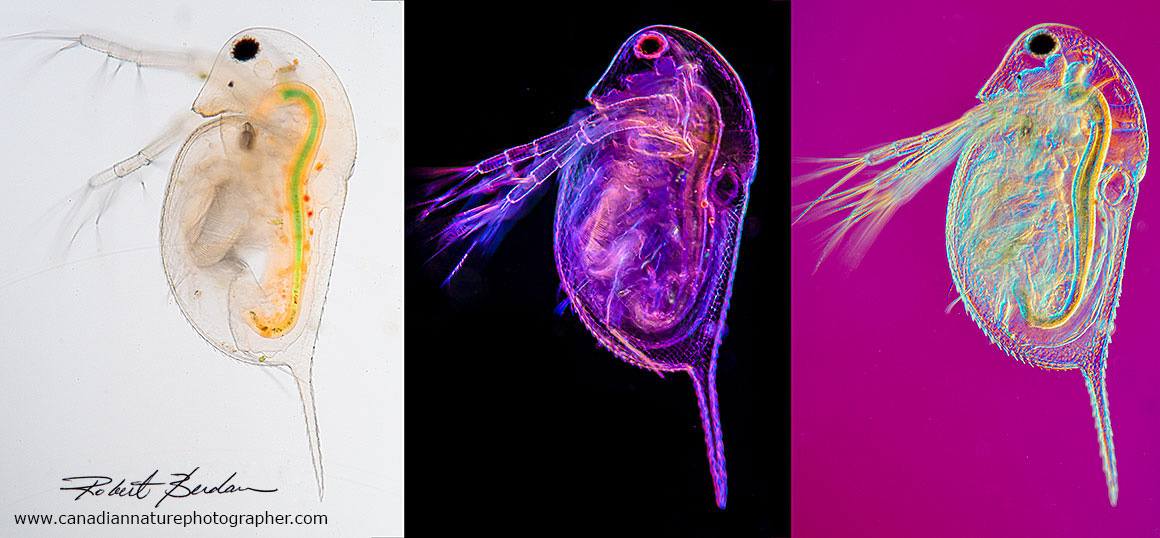
Daphnia photographed using three different microscopy techniques: brightfield microscopy, darkfield and Rheinberg, and DIC microscopy. 100X To learn more about Water fleas see my article on this web site.

Composite photo of two Daphnia using a combination of Darkfield and Rheinberg lighting. To learn about Rheinberg lighting see my article on this site. The filters can be made at home or purchased on Ebay for about $30. 125X
Adult Midge Fly

Chaoborus punctipennis, Family: Phantom Midges. It has spotted legs indicating it is a male, size: 4.5 mm long - photo courtesy of Wikipedia.
Mosquito larvae

Mosquito larva and a water flea (Daphnia sp). The water flea in this picture was safe as the mosquito larva is a filter feeder. Photographed with a Canon MP-E 65 mm macro lens, ring flash in a homemade mini-aquarium. I put a picture of a forest scene in the background beyond the depth of field of the lens and lit it with a LED flash light. Mosquitoes are closely related to Chaoborus midge flies, though Chaoborus will feed on mosquito larvae. 20X

Mosquito larva (Culcidae) called "wigglers" tend to be coiled in the water and hang below the water surface and breath from a posterior tube called a siphon. Mosquito larva are filter feeders. They also shed their skins four times before they pupate and then form adult mosquitoes. Chaoborus larva will feed on mosquito larvae. Stitched panorama, polarization microscopy about 50X. This is my artistic interpretation of a "disgusting" insect. Adult mosquitoes of some species kill more than 700,000 people every year by spreading infectious disease.

Adult mosquito on human skin taking a blood meal. The blood provides nutrients for the eggs. Mosquitoes in the Arctic that can't find a meal can still produce eggs, though not as many through a process of parthenogenesis. 100 mm macro lens.
Photography & Microscopy Techniques
There are various methods to photograph small organisms. The simplest method is to use a macro lens. Macro lenses come in different focal lengths: 60 mm, 100 mm, 200 mm. Macro lenses with larger focal lengths permit a larger working distance between the camera and the subject which can be an advantage if subjects sting or tend to fly away. For higher magnification imaging I use the Canon MP-E 65mm macro lens capable of up to 15X magnification. A microscope objective attached to a bellows can also be used to photograph images at high magnifications. I used the Canon MP-E 65 lens attached to a Canon 80 D APSC camera for some of the photos abover (APSC cameras use a smaller sensor that magnifies the image about 1.5X compared to a full frame camera). I used fiber optic lights and a Nite core flash light to provide back-lighting and facilitate focusing. A Canon ring flash with modelling lights was used stop the animals movement and allowed me to use apertures of F8 to F11 for greater depth of field. I also used the Canon lens on a tripod or a vertical stereoscope stand. To focus with this lens up close I had to move the camera and lens back and forth by sliding it on rails. I have a rail that can be focused by turning, but it isn't fast enough as the Chaoborus tends to wiggle about every 5-15 seconds which is barely enough time to achieve sharp focus and shoot.

The above photo shows a mini-aquarium I constructed from two glass plates separated by 1\8 inch waterproof foam (sometimes I used two layers of foam). I cut a rectangular slot in the foam in the top middle portion to create the mini aquarium. I use strong clamps to make a waster proof seal and 2 "L or corner" brackets so the glass stands upright. All the materials are available at local hardware stores for a few dollars. I also use a fiber optic light source and Nite core LED (Light Emitting Diode) flash light on a stand to provide a back light. If I want a green background I put a picture of a forest scene behind the glass and light it with the flash light.
This mini-aquarioum works well for small insects, diving beetles, mosquito and Chaoborus larva. For larger aquatic insects I use 2 layers of foam or I use a mini-aquarium I make from glass microscope slides held together by super-glue (not shown). The dimensions of the mini-aquarium is kept small so that organisms can't easily move out of focus (2 x 5 cm by 4 mm deep). It is still challenging to get aquatic insects sharp and in focus. I average about one good photo out of 10 taken - thankfully digital images does not cost extra as it did years ago with film.
With a black background I set exposure compensation to -1 or -2 F-stops for correct exposure. The other challenge is keeping the glass clean and free of dirt. I clean the glass with Windex, Q-tip swabs and remove bubbles and dirt digitally from the images using Adobe Photoshop. This photography setup also works for making videos but requires bright lights with the Canon MP-E 65 mm Macro lens.
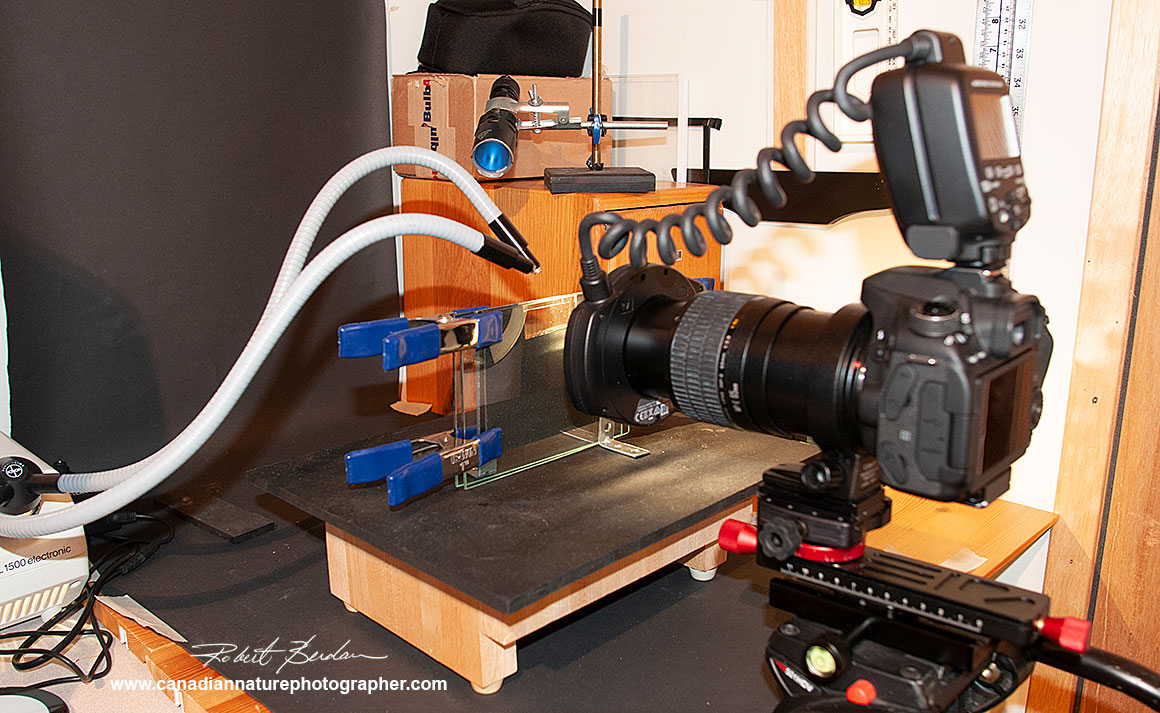
Mini-aquarium setup used for taking some of the photos shown above with a black background. I had to experiment with the position of the fiber optic and back lights to get the look I wanted.

Side view of the mini-aquarium set up. Sometimes the macro lens comes very close to the glass making it more challenging to find and focus on the specimens.

For larger insects like dragon fly larvae I use a vertical setup. They large insect larva are placed in a glass petri dish. For a white background I put an LED light below the dish; for a black background I use black felt or plastic under the dish. The size of the dish depends on the size of the organism I am photographing. With this set up it is possible to use focus stacking for live organisms if the organism stays still. If the organism moves continuously you can fix the organism in alcohol, but this may change its appearance. Chaoborus for instance turns an opaque white colour in 70% isopropanol.
Photography with Stereomicroscopes and Light Microscopes
Some stereoscopes have camera ports with adaptors for a DSLR camera and\or C mount dedicated microscope camera. The stereoscope below accepts both. I have a Nikon 7500 camera body attached with an economical ($100) Vitrox Ring flash from Ebay. This Flash can only be used in Manual mode with my Nikon and Canon cameras which is why I own a Canon ring flash which is more costly but can be used in automatic TTL (through the lens) metering mode. Zess offers a DSLR adapter for the Stemi that attaches to the C mount, but it is not parfocal with the eyepieces.

Above the Stemi stereomicroscope has two built in LED lights one at the back and one that lights the specimen from above. Either light can be used or both at the same time. I also use fiber optic lights.
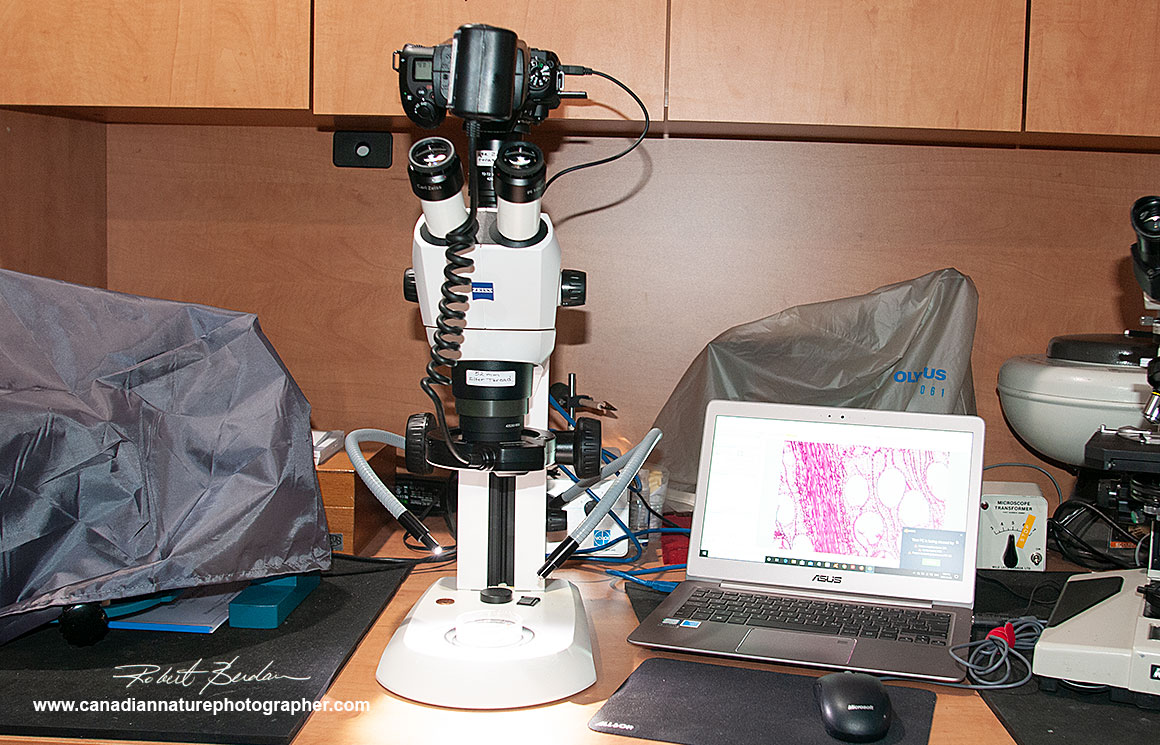
Above photo showing the Zeiss Stemi with Nikon 7500 DLSR attached above and a Vitrox ring flash. I also have a 0.5X reducer lens on the stereoscope to increase the working distance and decrease the overall magnification of the stereoscope. The camera can also be connected to a laptop computer via a USB cord and images are taken using Digicam software available free online.

Zeiss Axioscope microscope with a Nikon D800 camera attached. A USB cord connects the camera to my laptop and images are captured using Digicam control software. The photo tube is from Zeiss. This microscope provides differential interference contrast, polarization, dark field, bright field and phase contrast lighting.

Above is an older Nikon Optiphot that I refurbished. It has LED illumination for transmitted light and uses epifluorescence powered by a Mercury vapour lamp. I have a Nikon D500 attached to the photo tube that uses an Olympus NFK 2.5 and 5X photo-eyepiece. The photo adaptor is for Olympus cameras but I attach a Nikon extension tube to the camera and use black electrician tape to attach the camera and extension tube to the photo-tube. The camera is attached to my lap top via a USB cord so I can use the camera in LiveView mode with Digicam control software (available for free).
Summary
Chaoborus larva are fierce predators of plankton and they can look scary up close, but they are also fascinating to study when magnified. Their transparent cuticle makes studying their musculature relatively easy with a polarizing microscope. Chaoborus provides food for fish and some fishermen make a lure for fly fishing in order to try and catch fish. We don't have to worry about the Chaoborus adults or larval forms biting us in the water or the air, but they do form an important part of the food web. In some African countries huge swarms of Chaoborus midges are collected by local people to make kungu cakes, biscuits or burgers and are considered local delicacy rich in protein (Wikpedia). You can study these fascinating creatures with a magnifying lens and all you need is a few plastic jars to collect them and maybe some rubber boots. If you own a stereoscope or light microscope you see even more detail. I encourage you to watch some of the movies on links to YouTube provided below. If you would like to learn more about Chaoborus please read some of the reseach being done on this organism in my references. There are alien-like organisms on our planet, all you have to do is visit your nearby pond to collect and discover them. RB
References
Glassworm (2020) - Wikipedia
S. Kruppert et. al. (2019) Zooplankters' nightmare: The fast and efficient catching basket of larval phantom midges (Diptera: Chaoborus). PLOS one - PDF
R. Cockroft et. al. (2019) Emergence timing and voltinism of phantom midges, Chaoborus spp., in the UK - PDF.
L.C. Weiss et. al. (2018) Identification of Chaoborus kairomone chemicals that induce defences in Daphnia. Nature Chemical Biology: 14: 1133-1139. Web site.
T. Pattinson (2017) Phantom-midge, Chaoborus crystallinus. The Quekett Microscopical Club. - Web site. (Check Tony's books "The Freshwater Microscopist" on Blurb - they are excellent, written for amateur microscopists with numerous tips and excellent photography - I own four of his 5 books).
S. R. Dennis et. al. (2014) Endocrine regulation of predator-induced phenotypic plasticity. Oecologia DOI 10.1007/s00442-014-3102-8 - PDF
A. Borkent (2013) Insecta: Diptera, Chaoboridaes in Freshwater Invertebrates of the Malyasian Region - Research Gate PDF
P.J. Juracka et. al. (2011) Neckteeth formation in two species of the Daphnia curvirostris complex (Crustacea: Cladocera) J. Limnol., 70(2) Special Insert: 359-368, 2011 - DOI: 10.3274/JL11-70-2-20 - PDF
T.U. Berendonk et. al. (2009) Emphemeral metapopulations show high genetic diversity at regional scales. Ecology, 90 (10) 2670-2675. - PDF
C. J. Borkent and A. Borkent (2008) Description and phylogenetic interpretation of chromatophore migration from larval air sacs to adult structures in some Chaoboridae (Diptera). Can. Entomol. 140:630-640. - PDF
M. Lencioni (2006) Prey Selection by Chaoborus in the Field and Laboratory. J of Young Investigators - Web site.
A. Gosselin and L. Hare (2003) Burrowing behavior of Chaoborus flavicans larvae and its ecological significance. J. N. Am. Bentho. Soc. 22 (4): 575-581 - PDF
Chaoboridae in Australia - Web site
Chaoborus Taxonomy
Chaoborus punctipennis - educational Web site
D. Larson Zooplankton of the Great Lakes Chaoborus sp.- Web site
Chaoborus Videos
Chaoborus pinctipennis - Glassworm or Phantom Midge Larvae - YouTube
Spotted Salamanders, Spring Peepers, Phantom Midge Larvae - YouTube
Liquid lady (Cahoborus pupa) - How to make a Fly-fishing lure resembling Chaoborus - YouTube
Chaoborus larvae - YouTube
Chaoborus pupae - YouTube
Chaoborus pallidus pupa - YouTube
Chaoborus larvae in a flooded abandoned house foundation - YouTube
Equipment Resources
Motic - Source of stereo and light microscopes in North America - Motic North America.
Quality Microscopes source of Zeiss stereo microscopes in Calgary - qualitymicroscopes.ca
Digicam control software - Attaches your DSLR on your microscope to a laptop - free.
Note: Educators and students may use my images for reports and teaching, all other uses or for commercial use please contact me. If you use my images I appreciate attribution and a link back to my web site where possible. Note magnifications are approximate - 100X means taken with 10X objective, 200X with a 20X objective, 400X with a 40X objective.
Authors Biography & Contact Information
Bio: Robert Berdan is a professional nature photographer living in Calgary, AB specializing in nature, wildlife and science photography. Robert retired from Cell\Neurobiology research to pursue photography full time many years ago. Robert offers photo guiding and private instruction in all aspects of nature photography, Adobe Photoshop training, photomicrography and macro-photography. Portrait of Robert by Dr. Sharif Galal showing some examples of Robert's science research in the background.
Email at: rberdan@scienceandart.org
Web site: www.canadiannaturephotographer.com
Phone: MST 9am -7 pm (403) 247-2457.