
Pond Life Viewed with a Microscope II
by Dr. Robert Berdan
October 12, 2018

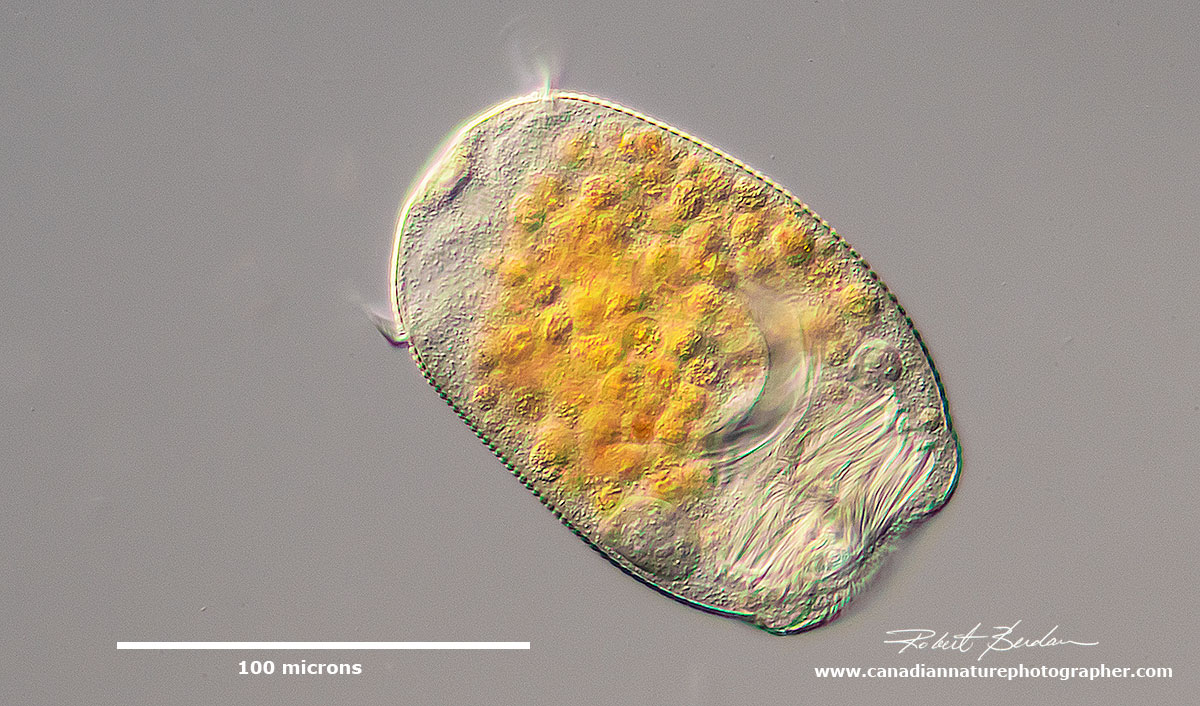
Stentor pyriformis with symbiotic algae (Chlorella). Heterotrich ciliates have a prominent adoral zone of membranelles circling the mouth which is used in feeding and locomotion, along with shorter cilia on the rest of the body.
Ciliates
Ciliates are among the most complex and diverse unicellular organisms on the planet that form an important component of aquatic ecosystems acting as predators and providing nutrition for organisms at higher trophic levels. Ciliates are also important in the treatment of sewage water and water purification (Madoni 2011). Originally ciliates were a subphylum within the protozoa, but now reside in the Phylum Ciliophora.
Ciliates have an elaborate cytoskeleton, cilia and two types of nuclei. One nucleus (macronucleus) functions in day to day processes within the cell, the other called a micronucleus (they can have more than one) functions in the exchange of genetic information. They range in size from 10 to 2000 microns. Over 10,000 species of ciliates have been described and it's been estimated that more than 30,000 species may exist. Ciliates are also notoriously difficult to identify and some require application of silver stains to recognize some species. Ciliates are found in any habitat that has water. For more pictures of ciliates see my previous article.

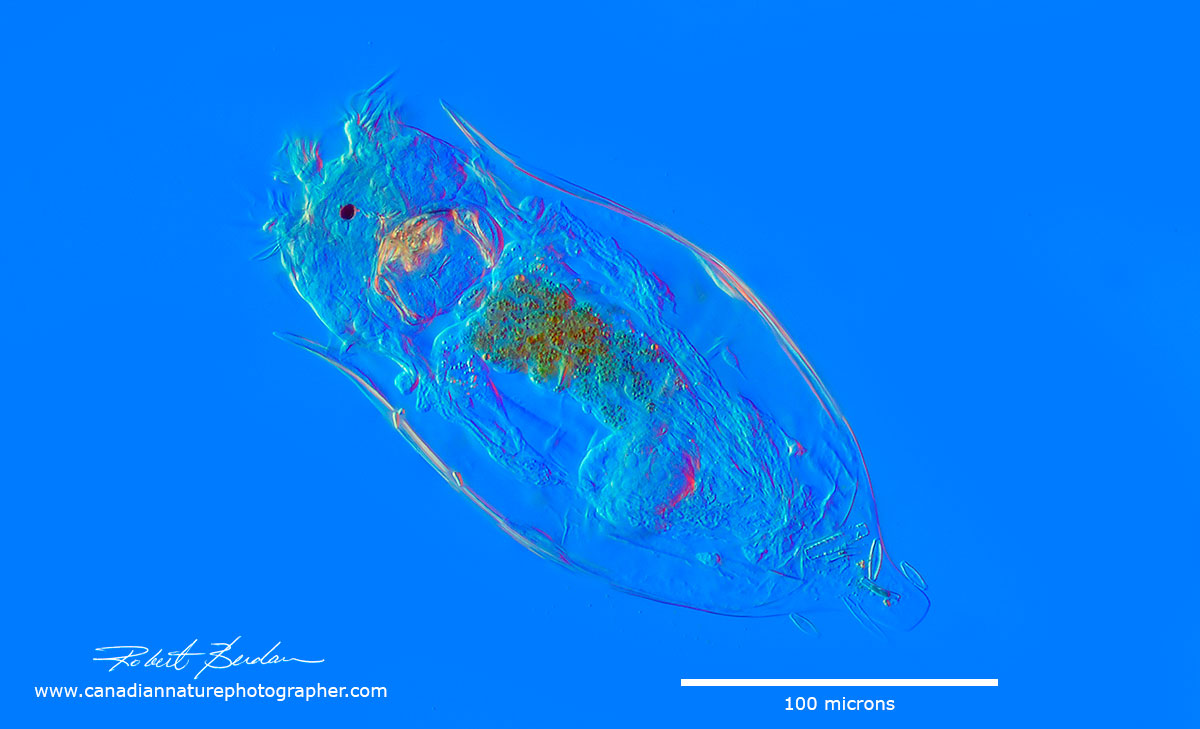
Pleurostomatid Amphileptus sp.? Elongate somewhat flattened ciliate with 2 prominent macronuclei and posterior contractile vacuole. (Can't rule out that this could also be Acineria or Litonotus)

Euplotes sp belongs to the ciliate order Euplotida whose species are characterized by rows of fused cilia called cirri on the ventral surface. Euplotes uses cirri (long spines) for swimming and to "walk" along the substrate. DIC microscopy. See video.
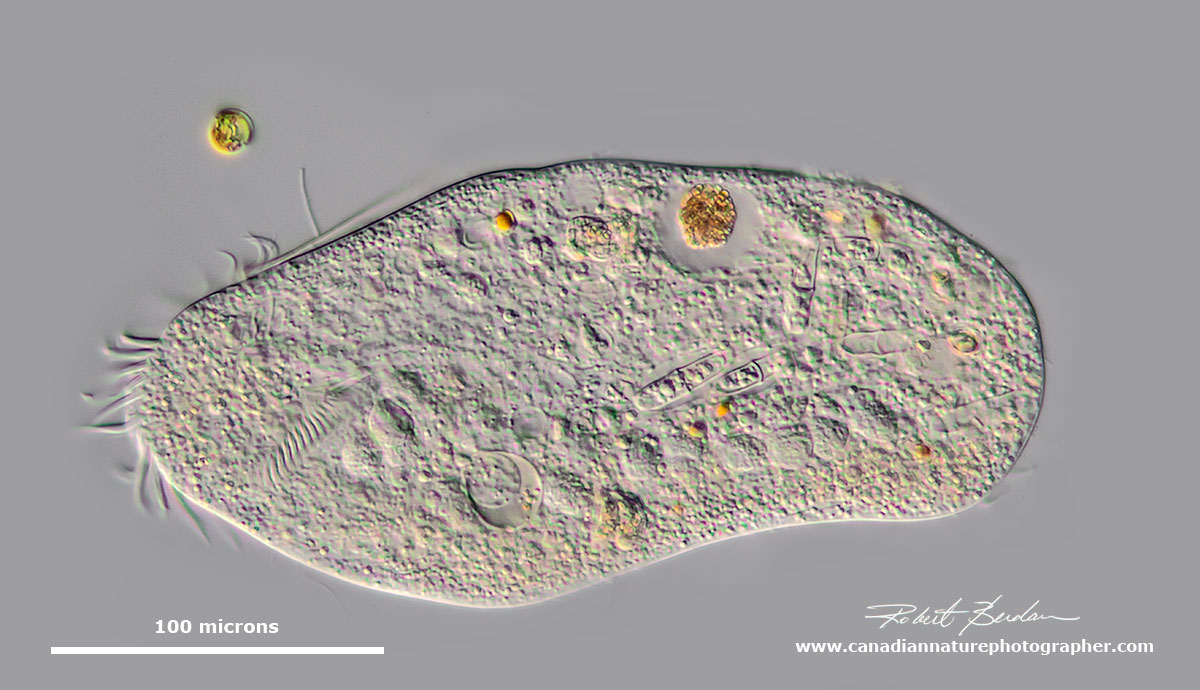
The depicted hypotrich might be a urostylid (e.g. Urostyla), but it is impossible to tell without seeing those ventral cirri. It preys on diatoms and filamentous algae. DIC microscopy.
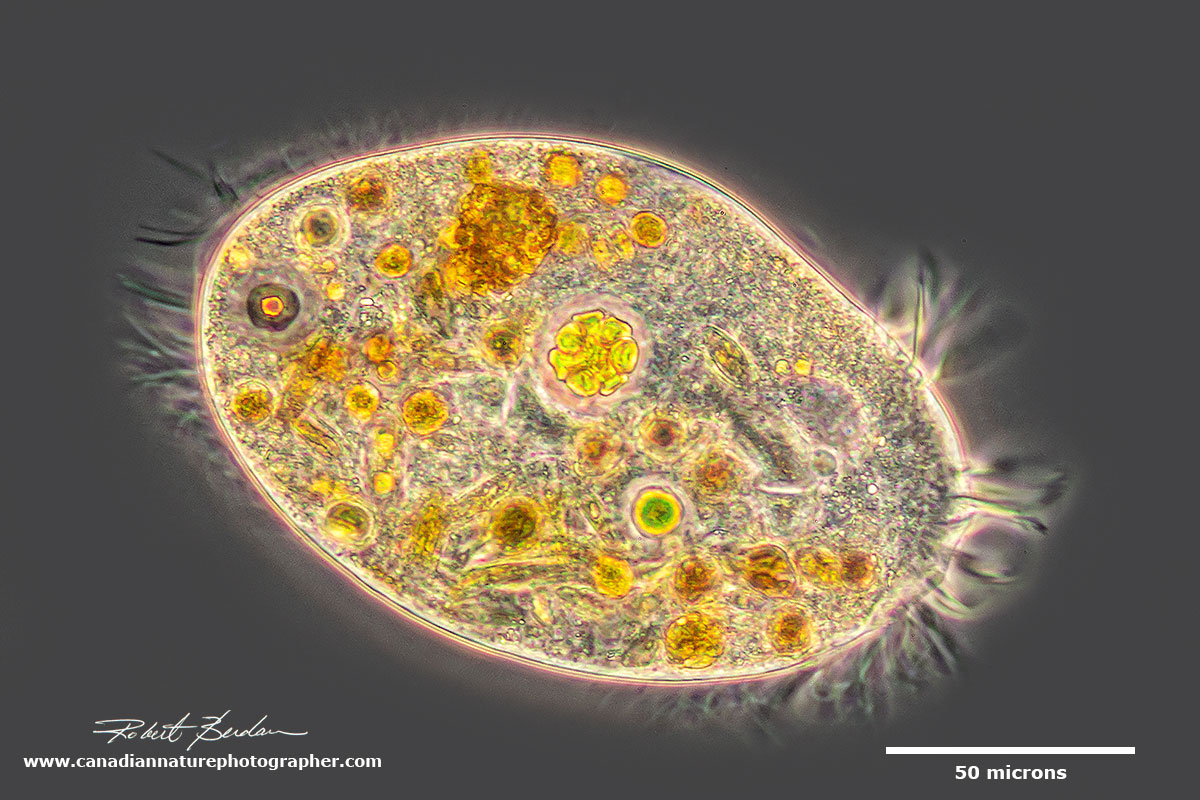
Hypotrich - they posses ciliary organelles called cirri which make up thick tufts of cilia. Phase contrast microscopy.
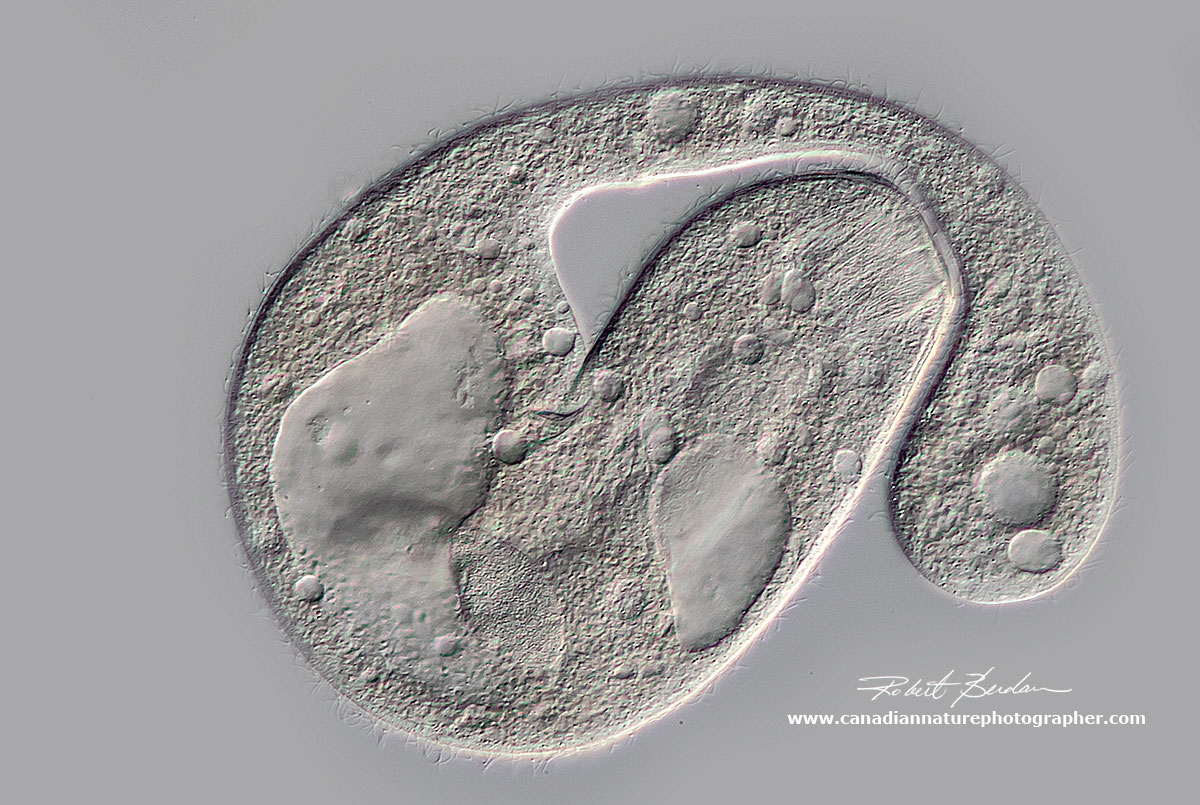
Unknown large ciliate with large macronucleus, several contractile vacuoles and internal filaments - oral nematodesmata on the apical end (near center of picture). This ciliate may be damaged. Possibly Holophyra member.

Relatively small ciliate with mouth on the apical end - unidentified species.

Hypotrich Stylonychia mytilus? DIC microscopy.

Hypotrich Stylonychia mytilus? DIC microscopy.

Hypotrich Stylonychia mytilus? DIC microscopy.

Ciliate DIC microscopy. Amphileptus procerus?

Trachelius ovum- this unusual ciliate ejects parts of its cellular contents when trapped by the coverslip in order to become a smaller organism. It's body appears to be very fragile and this may be a mechanism to escape predators.

Blepharisma lateritium (Order Heterotrichida) - Tear drop shaped pink ciliate. Their are about 40 different species of Blepharisma. Most have a red or pinkish colour caused by granules of the pigment blepharismin. There is a large contractile vacuole in the posterior end that is involved in osmoregulation. They feed on bacteria, flagellate algae, rotifers and other ciliates.
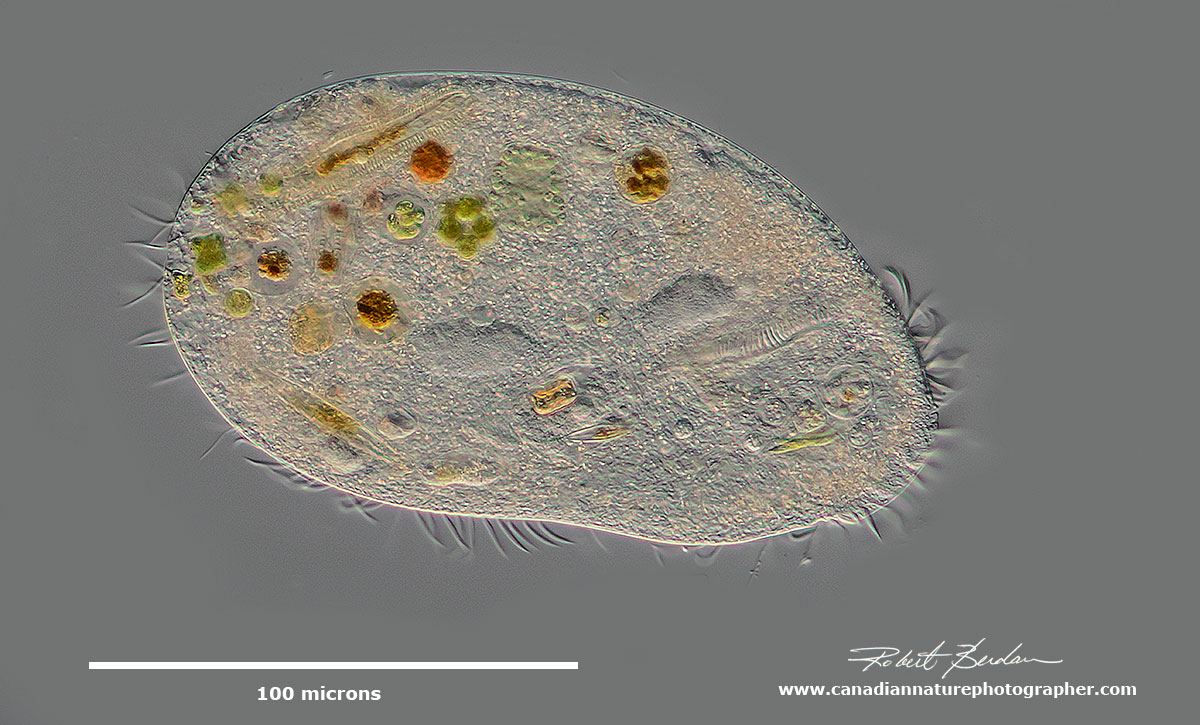
Hypotrich - unidentified ciliate. Hypotrichs possess compound ciliary organelles called "cirri," which are made up of thick tufts of cilia sparsely distributed on the ventral surface of the cell.

Closeup of ciliate showing diatoms inside and also one discus shaped diatom top right of the picture. DIC microscopy. About 400X.

Strange looking ciliate - possibly Paradileptus elephantinus? DIC microscopy. It is possible this ciliate may be deformed due to toxins in the water or damaged.
Peritrich Ciliates
In peritrich ciliates, cilia are restricted to a zone around the mouth. Many are sessile though they can form a motile telotroch stage and swim to another location. Some of the stalks attached to bells can contract quickly. Many also seem to grow in small groups or colonies on plants.

Vorticella convallaria? on a contractile stalk. Vorticella were first described in 1676 by Antonie van Leeuwenhoek. DIC microscopy.

Epistylid - they have a short stalk (Peritrich) - DIC microscopy

Peritrich ciliate Vorticella chlorellata or Pseudovorticella photographed from top view. They contain symbiotic chlorella inside. By attaching to some common debris as a colony they are too large to be ingested by some predators. DIC microscopy.
This is the swarmer (telotroch) phase of a peritrich ciliate - probably Vorticella sp. A sessile ciliate can turn temporarily into a motile one and move to a better place. When swarming, they shed their stalks, become somewhat cylindrical and develop a wreath of cilia at the posterior.
Rotifers
Rotifers are common in pond water especially around water plants. Rotifers have fascinated people for over 300 years ever since Antonie van Leeuwenhoek, a Dutch tradesman, began describing them. There are about 2000 species of rotifer. They are bilaterally symmetrical, eutelic (have constant number of cells) and are saccate to cylindrical in shape. On their anterior end they have a ciliated corona which propels them through the water and also brings food (bacteria and other small particulate matter i.e. detritus) into their mouth. In some species the corona has two concentric rings of cilia that beat resulting in the illusion of a rotating wheel - hence their name wheel-animacules. The second major feature of rotifers is a muscular pharynx (mastax) with chitinous jaws called trophi. These jaws can often be seen to move like a heart beat. The trophi are important in the classification of some species.
These organisms reproduce by hetergony a sequence of parthenogensis and sexual phases. In some species no males have ever been found. In fact most of the rotifers you are likely to see are females. The fact they propagate by parthenogenesis allows them to colonize new biotopes readily. When rotifers form cysts these can be carried by the wind. Others attach to birds feet and can travel to remote locations and I have even found rotifers in rain water from my gutters.
Rotifers range in size from 40 microns up to about 2 mm in length. The body consists of 3 parts: head, trunk and foot. On the segmented foot they often have spine-like feet with adhesive glands that allow them to attach to the substratum and crawl like an inchworm - see my movie on my article on Protozoa, Volvox and Rotifers.
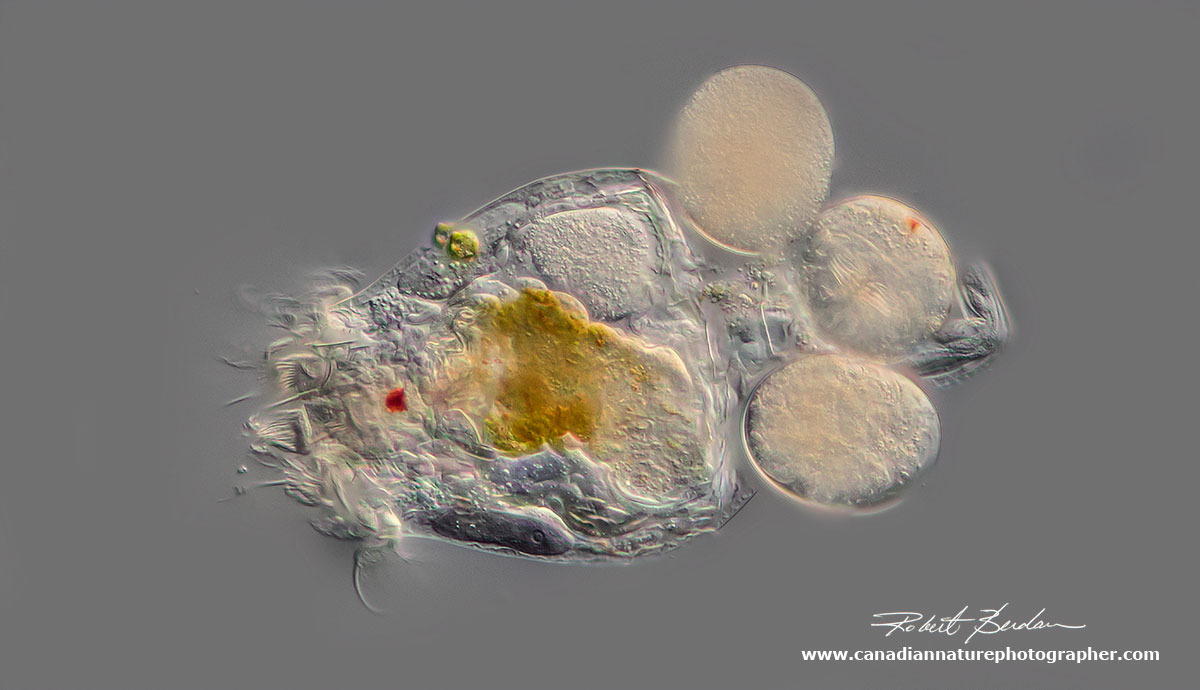
Rotifer carrying 3 eggs (Brachionus sp) about 400X DIC microscopy.
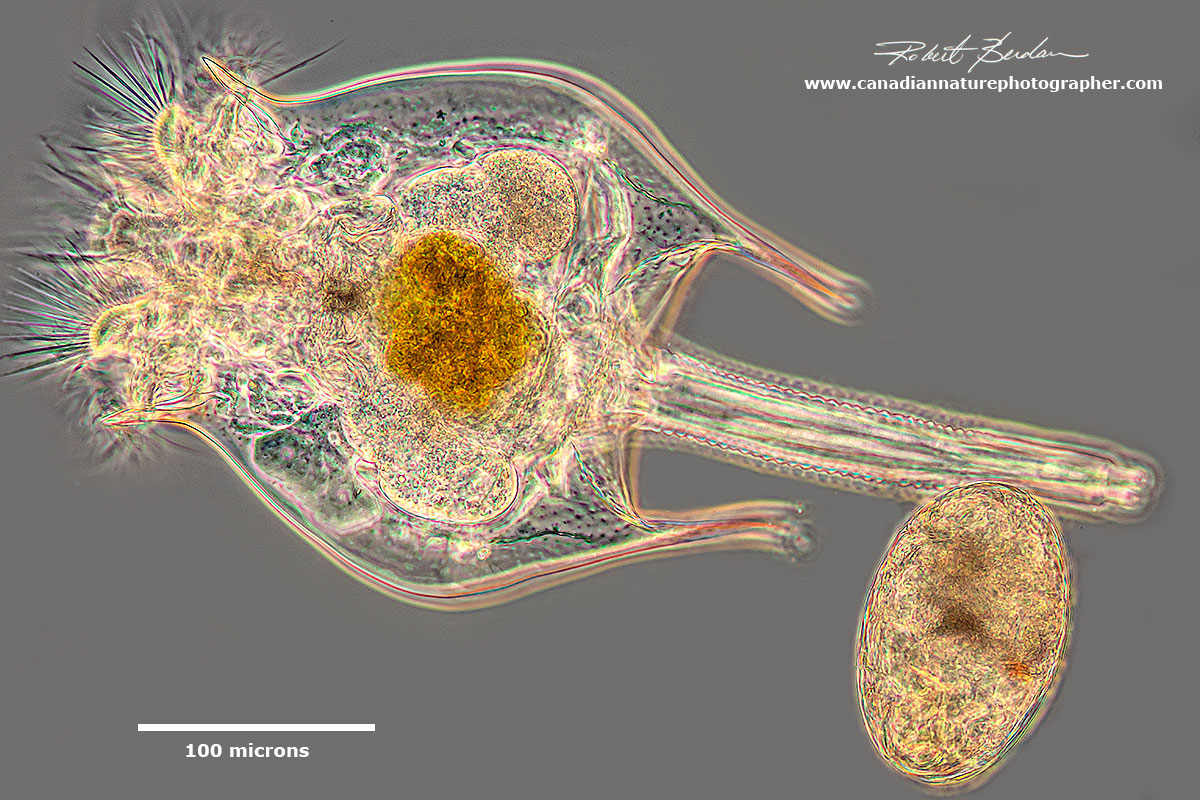
Rotifer Brachionus quadridentatus releasing an egg. This picture reminds of Alien in the movie. This rotifer was found in a storm drainage pond in Bowmount Park near my home.

Floscularia sp is a rotifer that lives in a tube and belongs to the subclass Monogononta. This animal can grow to 1.5 mm in length and often has eggs inside the tube which it can release. This rotifer is sessile and can attac hitself to the substratum or leaves of water plants.
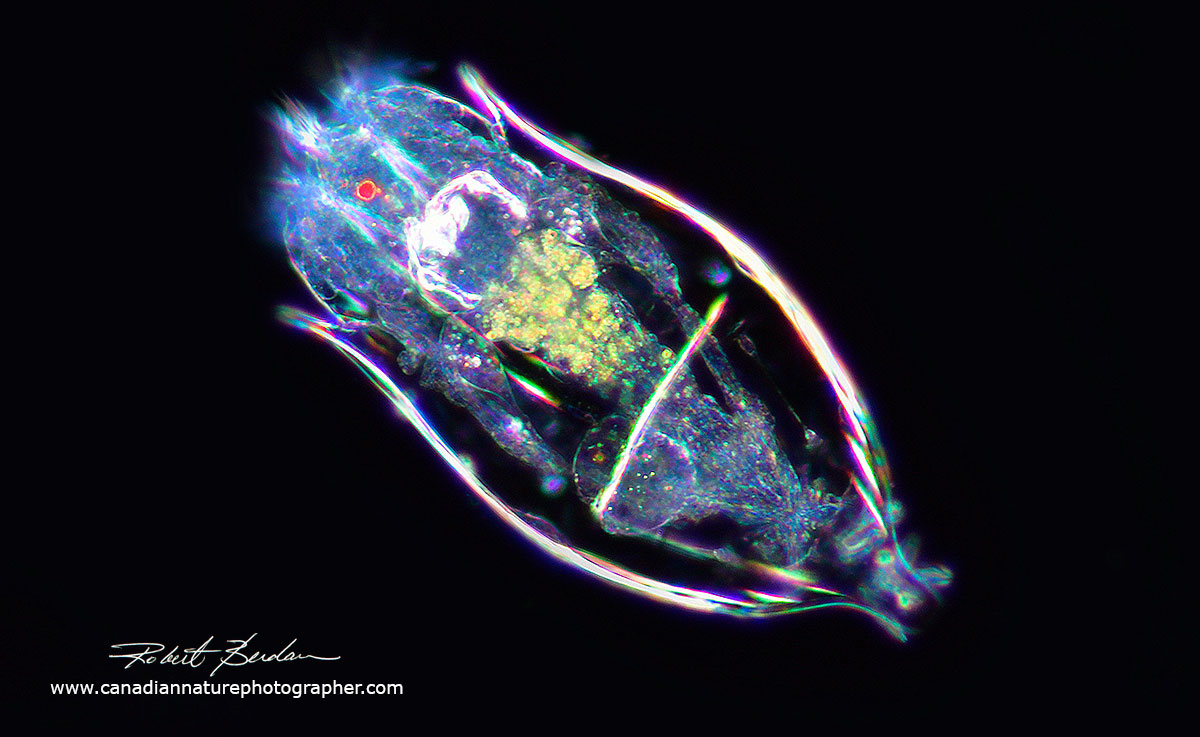
Notholca acuminata Found in both brackish water and eutrophic freshwater ponds. This one was found in storm drainage pond in Bowmount Park, near my home in Silversprings, Calgary. Darkfield microscopy. Note the single red eye.
Notholca acuminata DIC microscopy.

Side view of Brachionus sp of rotifer taken using phase contrast microscopy.

Bdelloid rotifer that was found in water associated with Lichen I collected in the Canadian Arctic. The inset shows a closeup of the trophi or chitinous jaws. DIC microscopy.

Above is the same Bedlloid rotifer but photographed using Darkfield microscopy.

Brachionus quadridentatus The outline-like image was not produced in photoshop, but by using a Phase I ring with the wrong 20X objective on my Axioscope. These rotifers are found in eutrophic freshwater ponds.

Brachionus quadridentatus view by Darkfield microscopy.

Brachionus quadridentatus viewed by Phase contrast microscopy.

Noctholca acuminata rotifer DIC microscopy

Noctholca acuminata? - rotifer photographed with phase contrast microscopy. Note the single red eye.

Asplanchnopus multiceps (alternatively Epiphanes clavulata ) is a large rotifer that can grow to 1 mm in size and can be seen with a stereo microscope. The U shaped structure inside the organism is the trophi or jaws. This rotifer was photographed using Darkfield illumination
Miscellaneous

Suctorian (Heliophyra minima) these are ciliates that become sessile and lose their cilia. The spines with knobs can contract and extend and may be used to ensnare and manipulate prey. I watched the organism as it extended and contracted its tentacles.

Heliozoans are organisms with a round body, no internal skeleton with radiating stiff pseudopodia and there are several groups (this one belongs to the Actinophryid group). The picture above shows Actinophrys. They are sometimes referred to as sun-animalcules. The spine-like axopodia radiate from their spherical bodies and are used to capture food. Actinospharium is related but larger organism (200-1000 microns) than Actinophrys and has many nuclei. Read more about the Taxonomy of Heliozoans.

Actinosphaerium eichhornii (identification courtsey Ferry J. Siemensma) - no spines and a highly vacuolated interior. DIC microscopy (actinophryids are known to lose axonemes in certain circumstances, such as elevated hydrostatic pressure, or following cold shock - Bruce Taylor personal communication).

Segmented worm called a Chaetogaster. They grow to to be several millimeters long. They are common in freshwater and are transparent. They have setae (bristles) on their ventral surface which gives them their name.
Photomicrography
For details on how I took these pictures please see my article - Tips on How to Take Better Pictures with a Microscope - Photomicrography. I used a Nikon D500 and Nikon D800 cameras, Zeiss Axioscope with DIC, Bright field, and Darkfield lighting, and controlled my cameras with free software Digicam control. Images were captured onto an Alienware laptop and processed in Adobe Photoshop. In many instances I needed to stack 2-12 images to increase the depth of field - for more information on this procedure see my article on focus stacking.
Note: I have attempted to identify the genus or species using various books and guides and have consulted one ciliate specialist (Bruce Taylor) for assistance, though any identity mistakes are my own. This information may be revised anytime I get more information and learn more about specific species.
References:
Bruce Taylor (2018) Web site - It came from the pond - resource about Ciliates.
Siemensma, F. J., Microworld, world of amoeboid organisms. World-wide electronic publication, Kortenhoef, the Netherlands. https://www.arcella.nl.
(2011) P. Madoni. Protozoa in wastewater treatment processes: A minireview. Italian. Journ. Zool. 78: 3-11.
(2004) N. G. Senler & I. Yildiz. Faunistic and Morphological Studies on ciliates from a Small Pond, with Responses of ciliate Populations to Changing Environmental Conditions - download PDF.
K. A. Mikrjukow and D. J. Patterson (2001) Taxonomy and Phylogeny of Heliozoa. III Actinophyrids. Acta Protozoologica. 40: 3-25 (PDF)
(2000) Illustrated guide to the Protozoa 2nd edition Society of Protozoologists. Allen Press, USA available on Amazon.com most recent Key to ciliates - check your library - excellent ref book.
(1999) W. Foissner, H. Berger and I. Schumberg. Identification and Ecology of Limnetic Plankton ciliates. Bavarian State Office for Water Management. 798 pages - download free ebook PDF
(1994) W. Foissner and S. Wölfl Revision of the genus Stentor Oken (Protozoa, Ciliophora) and description of S. araucanus from South American lakes. J. Plankton Res. 16: 225-289 - includes key to Stentors - PDF (see Dr. Foissner's other publications on his web site)
(1982) C. R. Curds British and Other Freshwater Ciliated Protozoa. Cambridge University Press.
(1974) A. Ruttner-Kolisko Plankton Rotifers - Biology and Taxonomy, Stuttgart, Germany
Authors Biography & Contact Information

Robert Berdan is a professional nature photographer living in Calgary, AB specializing in nature, wildlife and science photography. Robert retired from Cell\Neurobiology research to take up photography full time years ago. Robert offers photo guiding and private instruction in all aspects of nature photography and Adobe Photoshop training - including photomicrography, macrophotography.
1. Pond Life Viewed with a Microscope I
2. Photomicrography of Hydra - a model for studying regeneration and aging
3. Photographing Daphnia
4. Photographing Gastrotrichs
5. Photographing Rotifers
6. Photographing Ciliates
7. Photographing Stentors - A Large Unicellular Protozoan (ciliate) living in Freshwater
8. How to Collect and Photograph Water Bears (Tardigrades).
9. Tips on How to take Better pictures with a Microscope
10. Microscopic Pond Organisms from Silver Springs Calgary
11. Microscopic Life in Ponds and Rainwater - Pond Scum I
12. Photographing Microscopic Plant and Animal Life - Pond Scum II
13. Photomicrography and Video of Protozoa, Volvox and Rotifers
14. Home Microscopy Laboratory for Photomicrography
15. The Art & Science of Photomicrography with Polarized Light
16. Photographing Through a Microscope Photomicrography - Inner Space
17. Focus Stacking comparing Photoshop, Helicon Focus and Zerene
18. Rheinberg Filters for Photomicrography
19. Scanning Electron Microscopy - Photography
20. Photomicrographs of Diatoms from 1877 by John T. Redmayne
Email at: rberdan@scienceandart.org
Web site: www.canadiannaturephotographer.com
Phone: MST 9am -7 pm (403) 247-2457.