
The Science & Art of Wine Crystals by Polarized Light Microscopy - Abstract Art
by Dr. Robert Berdan and Brandon Berdan
February 24, 2023 (updated November 2, 2023)

Red wine crystals made from Napa Valley from Kia Behnia's vineyard 100X.

Crystals from Chardonay wine from a sample pack from a Niagara Escarpment winery. The above image is a panorama of several images stitched together. Polarized light microscopy 40X.
Introduction
The production of a good wine requires a combination of science & art. Wine contains crystals of tartaric acid under certain conditions can produce beautiful images that resemble abstract art when viewed with a polarizing light microscope. Here we show some of these crystals and provide a brief overview of Louis Pasteur's early studies of tartaric acid that lead him to the discovery of Chirality. This discovery had important consequences in chemistry and medicine and it has been suggested that Pasteur's early interest in art contributed to the discovery (J. Gal, 2008).
Louis Pasteur (1822- 1895)
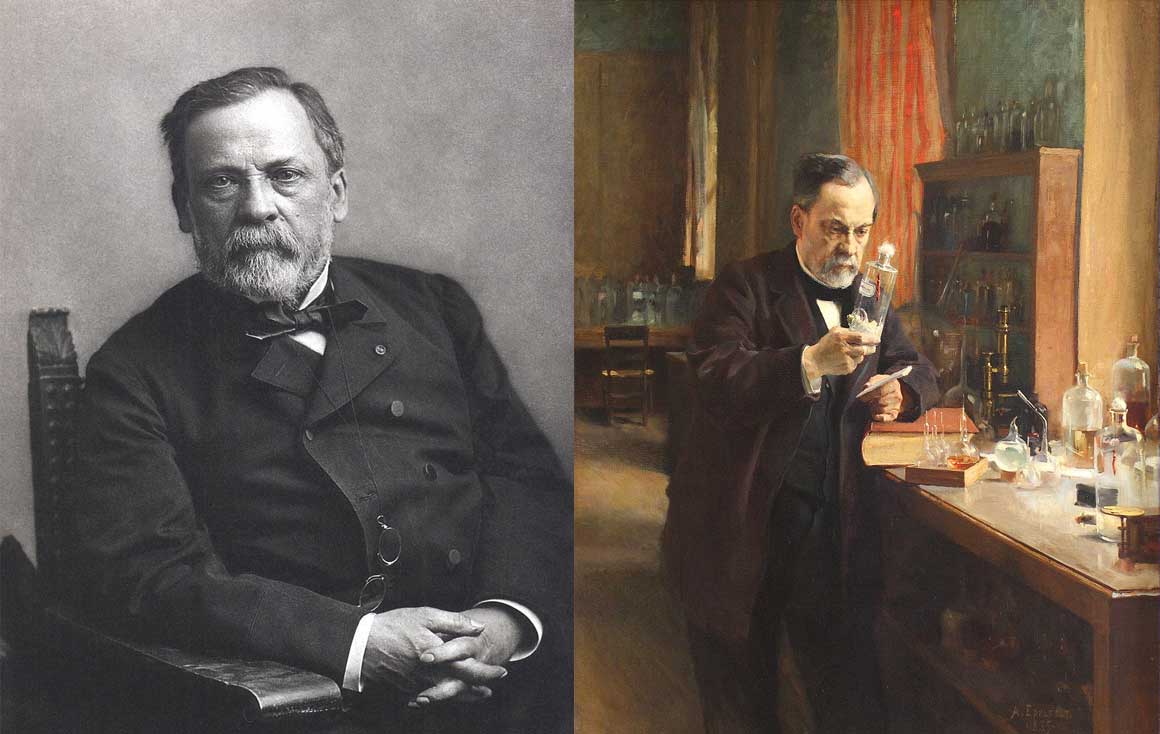
Left: photo of Louis Pasteur by Paul Nadar in Dole France. Right: painting of Louis Pasteur in his laboratory by Albert Edelfelt in 1885. Images courtesy Wikipedia commons.
Louis Pasteur was one of the earliest scientists to study wine crystals. He also became one of the most influential scientists in the past two centuries Wikipedia. Pasteur was a French Chemist and Microbiologist whose vaccines saved millions of lives. His first major discovery began with the analysis of tartaric acid crystals from wine. He discovered tartaric acid could form two different crystals that were mirror images called enantiomers. Enantiomers are pairs of compounds with exactly the same physical properties, boiling point, melting point but the crystals and their molecules can have opposite three-dimensional shapes. Pasteur made the discovery at the age of 25 just 8 months before receiving his doctorate. Chirality plays a role in the binding affinity and interactions between some drugs and its target, thus shaping a drug's pharmacology. For this reason, in 1992 the Food & Drug Administration (FDA) outlined a series of guidelines for the pharmaceutical development of single enantiomers and racemates.

Tartaric acid crystals from wine by polarized light microscopy 100X.
Chirality is a property of symmetry; an object or a system is chiral if it is distinguishable from its mirror image and not superposable on its mirror image. It turns out that other molecules and some drugs exhibit chirality. One of those most notorious substances is Thalidomide. Thalidomide was used in the late 1950’s to treat morning sickness in pregnant women. Thalidomide was initially thought to be safe for women during pregnancy; it was a disaster and caused more than 10,000 children to be born with a range of deformities and caused thousands of deaths. It was introduced without being tested on pregnant women. Thalidomide exists in two mirror-image forms: it is a racemic mixture of (R) - and (S)-enantiomers and the (S)-enantiomer is teratogenic. A teratogen is anything a person is exposed to or ingests during pregnancy that's known to cause fetal abnormalities. Thalidomide was removed from the market in 1961 (Wikipedia).

French Red wine crystals by polarized light microscopy 100X.
Tartaric acid is found naturally in grape juice and wine. The tartaric acid appears to deter predators (insects and other animals) from eating the grapes. Tartaric acid crystals can be seen in wine after it precipitates out during fermentation and bottling of wines. It also forms crystals on the bottom of some wine corks used to seal bottles. When tartaric acid precipitates in the bottom of a wine bottle or cork, it is referred to as “wine diamonds”. The crystals can be induced to precipitate by increasing the alcohol content, altering pH or by chilling the wine before bottling. Wine diamonds are not harmful to injest, and contributes to the flavour and pH of wine. Some people don’t like its appearance so some wineries precipitate the crystals by chilling the wine before bottling. The two enantiomers of tartaric acid are not harmful, and in fact Crème of Tartar which is used in baking, is a mix of the two enantiomers.

Above is a polarimeter made by Bellingham & Stanley Ltd. This optical tool was made in England around the late 1800s - source Di Mano in Mano. The polarimeter is used to measure how some chemical solutions rotate the plane of polarized light. The polarimeter was first used as a chemical instrument by Jean Baptiste Biot around 1816. Biot was a mentor of Louis Pasteur.

Schematic of a polarimeter showing the principles behind its operation. Unpolarized light is passed through a polarizing filter before travelling through a sample in solution. The degree of rotation of polarization is determined by a second, rota-table polarizing filter. Operating principle of an optical polarimeter. 1. Light source 2. Unpolarized light 3. Linear polarizer 4. Linearly polarized light 5. Sample tube containing molecules under study 6. Optical rotation due to molecules 7. Rotatable linear analyzer 8. Detector. Diagram courtesy Wikipedia Creative Commons.

Above is a modern Polarimeter used in a student chemistry lab at the University of Calgary

Tartaric acid molecules showing the two enantiomer structures found in a racemic mixture.
In 1820 Philippe Kestner, an industrial chemist, had prepared a mysterious form of tartaric acid from vinification (winemaking) that was called para-tartaric acid. It’s properties were identical with that of natural tartaric acid (bi-potassium tartrate) found in grapes but in aqueous solution the para-tartaric acid did not rotate the plane of polarized light. Kestner offered Pasteur a sample for study. Pasteur began to analyze its properties and noticed that the paratartrate was made up of two types of crystals that were mirror images.

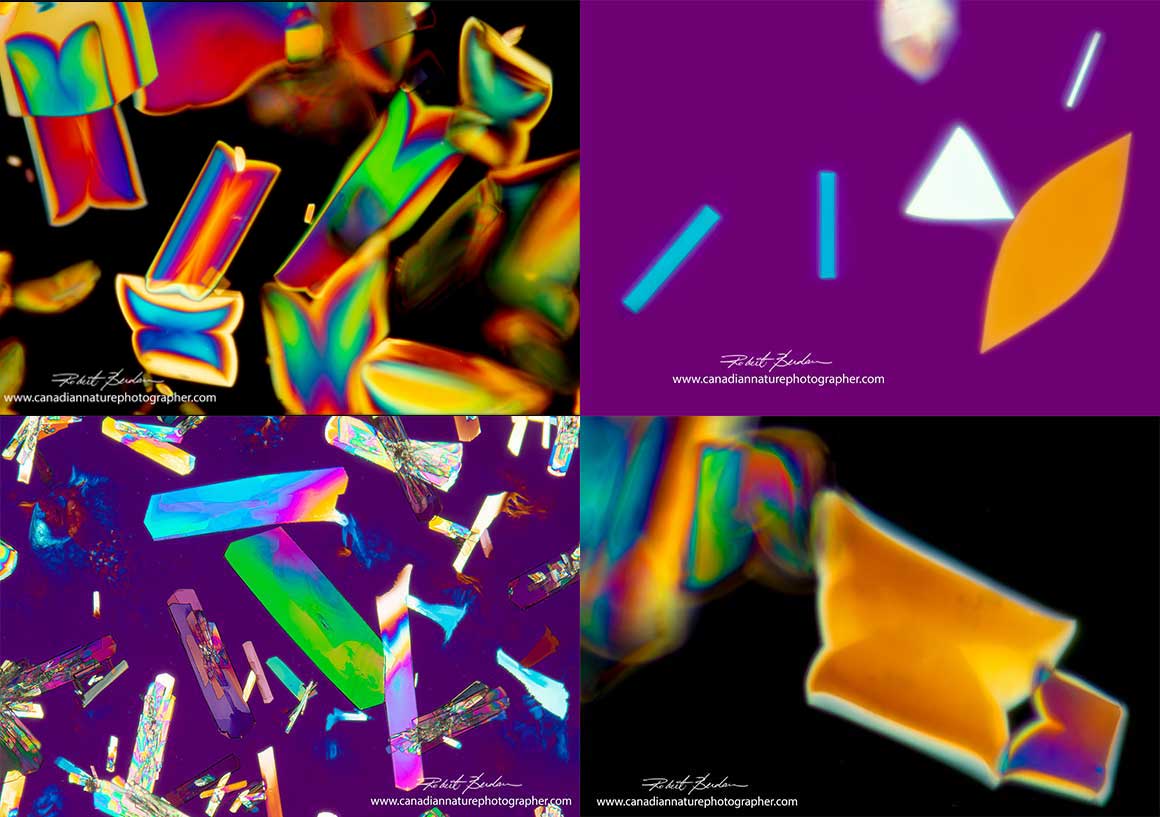
Tartaric acid crystals by polarized light microscopy 200X
Pasteur managed to separate the two different shaped crystals using tweezers and a magnifying lens. The two crystal forms of tartaric acid were made by boiling the natural form of tartaric acid. Pasture noticed two types of crystals in the racemic mixture were geometric mirror images see below. Natural tartaric acid made in grapes rotated polarized light to the left in a polarimeter. The racemic mixture, however had no effect on the plane of polarization in the polarimeter but other wise had identical properties. After isolating the "left" and "right" shaped crystals and dissolving each one in water, Pasteur tested them in the polarimeter and found that one rotated the plane of polarized light to the left and one to the right. When the crystals were dissolved together into a solution in equal amounts they did not rotate plane polarized light but cancelled the effects of each other. A racemic mixture is denoted by a prefix (±)- or dl- (for sugars a prefix dl- may be used), indicating an equal (1:1) mixture of dextro and levo isomers.

These crystals above shown in Fig. 1 and Fig.4 are from one of Pasteur's original publications. The bottom two crystals are a modern diagram of these two crystalline configurations.

Thus Pasteur showed that para-tartaric acid was a mix of the two crystals called a racemate or racemic mixture. A racemate is a mixture of equal quantities of two enantiomers, that have dissymmetric molecular structures that are mirror images of one another. This important observation and discovery became the foundation of molecular dissymmetry in chemistry even though the structure of tartaric acid was not known in Pasteur’s time.
The question is why did Louis Pasteur at the beginning of his career discover this when so many other prominent scientists were not able. Joseph Gal (2008, 2017), a modern day chemist, put forth a thesis that Pasteur’s early interests and experience with art was the reason that he noticed the two mirror shaped crystals while others did not.

Paintings by Louis Pasteur of his parents Left: Jean-Joseph Pasteur 1842 and de Jeanne Étiennette Roqui 1836. Courtesy Wikipedia creative commons.
Pasteur’s artwork was reproduced using lithography. Lithography produces mirror images of the art. Pasteur would sometimes make portraits appear more natural by viewing his subjects in a mirror while painting or drawing them. The resulting prints produced by lithography then made a mirror image of the artwork that was more life-like. Canadian painter Robert Bateman would also sometimes paint pictures viewed in a mirror and\or he checked his compositions in a mirror.
Pasteur‘s research went on to disprove spontaneous generation theory where it was thought at the time that microbial life could appear spontaneously. He showed that microbial life did not appear if the solutions were first boiled (sterilized). Further studies led to the technique named after him, Pasteurization where wine, beer and milk’s storage life could be extended by killing pathogenic bacteria and those causing spoilage. Pasteur's research also led to the development of new vaccines that saved millions of lives. He made vaccines to fowl cholera, rabies, and anthrax (Science History Institute 2017 – Louis Pasteur).
Tartaric Acid Crystals

Tartrate crystals on a cork of a white Chardonnay wine. Photo by Francesco Santini Wikipedia commons.
In this article we show images of tartaric acid crystals from different types of wine that we have modified to form crystal sheets. The crystals were photographed with both Nikon and Canon DSLR (digital single lens reflex) cameras viewed with a polarizing light microscope (Motic BA 310 and Zeiss Axioscope A1)
. We tried freezing wine, drying wine on microscope slides, heating it, altering the pH, modifying the cations calcium, sodium, potassium and ammonium. We
have also tried to make the enantiomers of tartaric acid crystals. We also added components to wine that are used in the fermentation of wine – see J.A. Considine, L. Dip, and E. Frankish (2014), P. Ribereau-Gyon, Y. Glories, A. Maujean and D. Dubourdieu (2006). For a description of the techniques and methods used to make crystals see (R. Berdan, 2017, R. Berdan, 2019, R. Berdan 2021, R Berdan 2022, R. Berdan, 2022 on this web site).
Photography of Wine Crystals.
We started by examining grape skins in the microscope and we could see crystals in the grape skin which are presumed to be tartaric acid.

Shown above are crystals in grape skin examined with polarizing light microscope 200X
We also examined tartaric acid crystals in "Creme of Tartar" which is a racemic mixture of the crystals. We dissolved the powder in different solutions and heated them on microscope slides. The crystals formed rhomboid shapes. After varying pH of a solution of creme of tartar some crystals formed feather-like edges.

Tartaric acid crystals from creme of tartar by polarized light microscopy 100X.
Tartaric acid crystals from creme of tartar by polarized light microscopy. Under certain conditions the crystals develop feather-like edges (see below).

Tartaric acid crystals with feathery edges viewed by polarized light microscopy 100X.

Tartaric acid crystals dissolved in ethanol by polarized light microscopy100X.

Tartaric acid crystals by polarized light microscopy 100X.

Above is a photomicrograph of tartaric acid crystals that formed around the edge of a drop of red wine heated on a microscope slide. 50X.

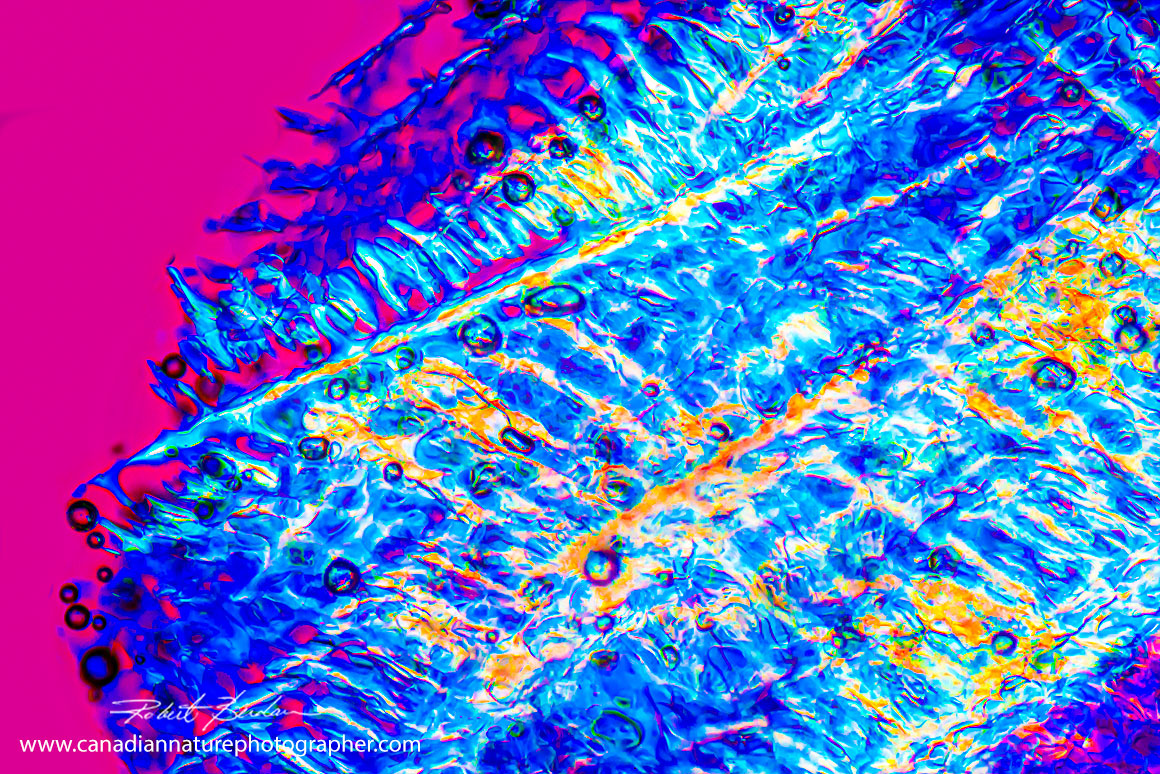
Tartaric acid crystals formed sheets. Polarized light microscopy 100X

Tartaric acid crystals formed sheets under certain conditions in red wine - viewed by polarized light microscopy 100X
After experimenting with tartaric acid crystals we sampled a wide variety of different wines from local stores and ordered a small box of wine with nine 50ml samples from Icellars. We tasted the wines before trying to make wine crystals (just for fun). Getting large crystals to form is challenging and required a variety of different procedures.
We began reading about the chemistry of wine making to get a greater understanding of the processes used (A. Considine, L. Dip, and E. Frankish, 2014). We found that some wines were better then others for making crystals and that there was a great deal of variability in crystal formation as shown in the images.
Making Wine crystals by Freezing
One of the first things we did to make crystals of wine was to freeze small samples on a microscope slide at -20 C. We knew that freezing water or beer forms crystals that exhibit different colours with a polarized light microscope (R. Berdan 2019).
Frozen white wine crystals 100X. Notice the air bubbles that form when the wine melts.
Frozen red wine crystals from Napa Valley 100X.
Frozen crystals melt quickly at room temperature and melting is accelerated by light and heat from the microscope light source. It was necessary to find a region of the frozen crystals to photograph quickly before they melted (usually less than a minute). Alternatively one could put a microscope in a cold room or outside in a garage during winter in Canada. This technique works for photographing snowflakes. The results were interesting but frozen crystals did not display the complex forms and shapes we were looking for.
We also tried cooling wine samples in a fridge to precipitate the wine crystals and examine them. This worked well especially with white wines and the cooling process is used by the wine industry to reduce the occurrence of wine crystals in the bottles. Wine crystals are primarily made up of tartaric acid. Additional techniques were used to precipitate wine crystals including altering pH, adding ethanol, adding seed crystals and other chemicals. Below we show some of our favourite wine crystal images.
Wine Crystals formed by drying samples on microscope slides
Below are just a few of the hundreds of images we produced from different wines. Modifying our methods resulted in a variety of different crystal shapes.

Crystals from red wine sample (CA1) from Napa Valley. The crystals exhibit feathery or snowflake-like shapes - polarized light microscopy with full wave retardation plate 100X.

Above crystals from Sauvignon blanc wine sample from the Niagara Escarpment 100X.

Chardonay from the Niagara Escarpment crystals by polarized light microscopy 100X.

Red Wine from Napa Valley by polarized light microscopy 100X

French white wine crystals by polarized light microscopy 100X.

French red wine crystals by polarized light microscopy 100X.

French rose wine crystals by polarized light microscopy 100X.

Red wine crystals (CA1) from Napa Valley by polarized light microscopy 40X.

Red wine crystals by polarized light microscopy 100X.

Red wine crystals by polarized light microscopy 100X.

Red wine crystals by polarized light microscopy 100X.

Rose wine crystals by polarized light microscopy 100X.

Red wine crystals from Napa Valley by polarized light microscopy 100X.

French white wine crystals by polarized light microscopy 100X.

French white wine crystals by polarized light microscopy 100X.

Red wine crystals from Napa Valley (CA2) 100X.

Red wine crystals from Napa Valley by polarized light microscopy 100X.

Red wine crystals by polarized light microscopy 100X

French red wine crystals by polarized light microscopy 100X.

Wine crystals by polarized light microscopy 100X.

Red wine (CA1) crystals Napa Valley polarized light microscopy 100X.

Red Wine (CA1) crystals Napa Valley by polarized light microscopy 100X.

Red wine (CA1) crystals Napa Valley by polarizing light microscopy 100X.

Red wine crystals from Napa Valley by polarized light microscopy 100X.

Red wine (CA1) crystals from Napa Valley polarized light microscopy 100X.

Red wine (CA1) crystals from Napa Valley by polarized light microscopy 100X.

Red wine (CA1) crystals from Napa Valley polarized light microscopy 100X.

Red wine (CA1) crystals from Napa Valley by polarized light microscopy 100X.

Pinot Noir wine sample from the Niagara Escarpment by polarized light microscopy 100X.

Red wine by polarized light microscopy 100X

Red wine by polarized light microscopy 100X
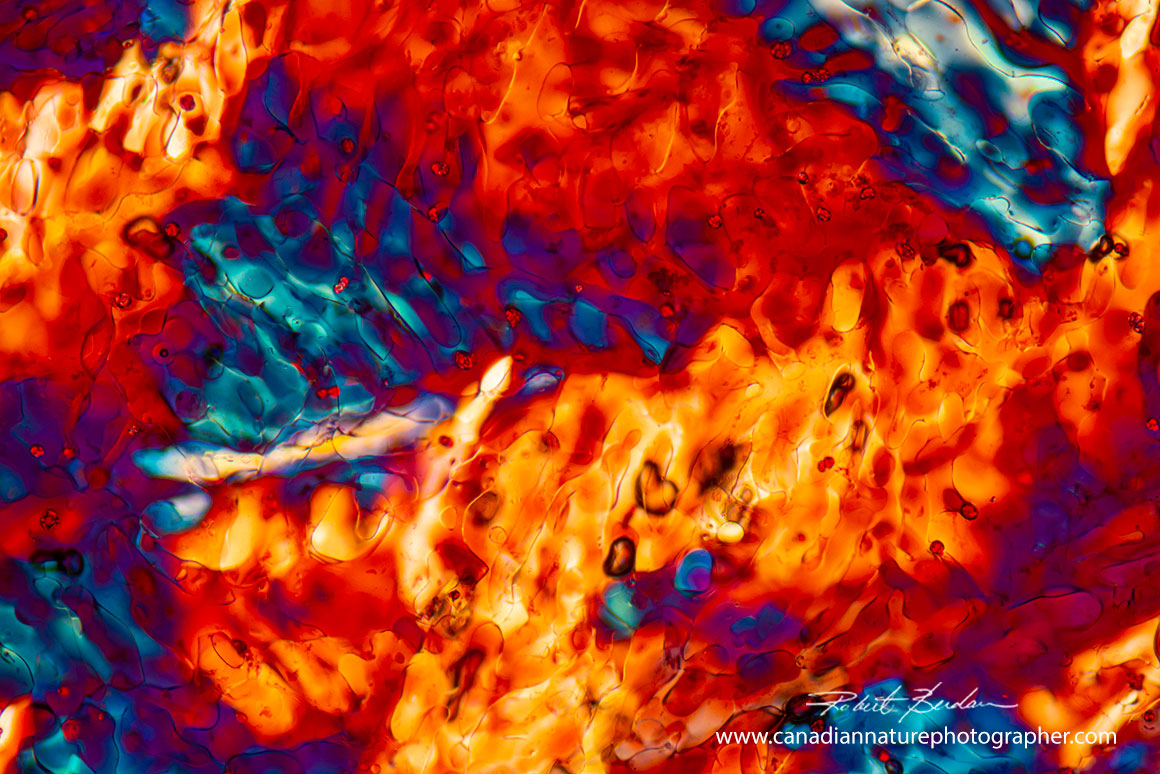
The images above show the wide variation in the shapes and forms that wine crystals can exhibit under different conditions. The large array of colours is due to the ability of a polarizing microscope to produce interference colours in birefringent substances such as crystals.
One of the most challenging tasks for the photographer is selecting attractive compositions. In most instances one can change the magnification, orientation of the crystals, or filters to modify the colours. Many of our preparations and experiments did not result in crystals and that is where knowledge of a bit of science and chemistry is helpful.

Red wine crystals by polarized light microscopy 50X.
Wine crystals from different wines such as a red, white, and rose are not easy to tell apart. Tartaric acid crystals, however are often distinctive in shape. We recommend first experimenting with tartaric acid crystals and changing the conditions under which the crystals form. If you want to make wine crystals read some of the scientific papers in our reference section and be prepared to experiment as that is what scientists like Louis Pasteur and we did. It can be frustrating at times, but it can also be rewarding when you discover methods that work.

Red wine crystals viewed by polarized light microscopy 100X.
Whether or not images of wine crystals constitute abstract art is open to conjecture. According to Wassily Kandinsky, abstract art is art that does not attempt to represent an accurate depiction of a visual reality but instead uses shapes, colours, forms and gestural marks to achieve its effect. From our perspective we hope the crystal images evoke positive emotions, especially while you are enjoying your favourite wine. We believe these images could make attractive wall hangings or for commercial use - please contact us if interested.

Chardonay crystals by polarized light microscopy 100X.

Red wine crystals by polarized light microscopy 100X

Crystals of red wine from Napa Valley by polarized light microscopy 100X.
Summary
Louis Pasteur in the 1800's made a significant discovery that tartaric acid from wine could exist as two enantiomers which established a new field in chemistry. He went on to produce methods (pasteurization) which extended the life of wine, beer and milk allowing these beverages to be preserved longer and allowed wine to be shipped long distances. In this study we show that tartaric acid crystals in wine can form beautiful shapes when examined with a polarizing light microscope. We also show that certain treatments of wine permit the tartaric acid to form crystal sheets which can resemble flowers and abstract art. Finally we try to show that both science & art can be complementary ways of looking at the world.
Acknowledgements
I wound like to thank Kia Behnia and Tracy Borman for sending me samples of their red wine from their Napa Valley vineyard. The samples we received were still being processed and mixed for launch of their new wine this spring. Kia is also interested in promoting art and music and provided financial assistance for this project.

Photo of Kia Behnia and Tracy Borman in their vineyard in Napa Valley, California. Photo by Allison Watkins. Read about their vineyard. All pictures labeled with Napa are wine samples from their vineyard.
Neotempo.wine web site featuring Art and Science
Watch YouTube video Phytech Grower Testimonial: Kia Behnia with scenes from their vineyard.
https://www.youtube.com/watch?v=geq1CE7ErHA&t=1s
Brandon Berdan is a 4th year chemistry student at the University of Calgary. He helped with experiments, took photos, and proofed the article. Donna Berdan also proofed the document.
If you might be interested in purchasing images for prints, advertisements etc please contact Robert Berdan. Some of the wine images are being used to add labels on wine bottles.
Note: Educators and students may use my web images for reports and teaching, all other uses or for commercial use please contact me. If you use my web images for educational use we appreciate attribution and a link back to this web page.

Red wine crystals by polarized light microscopy 100X
References & Links
Louis Pasteur biography, art and pictures by Wikipedia (2023)
Works of Pasteur - Pasteur, Louis (1822-1895) Auteur du texte. Oeuvres dep Pasteur. Tome 1/ reunites par Pasteur Vallery-Radot .. 1922-1939. https://gallica.bnf.fr/ark:/12148/bpt6k7356c.r=oeuvres+de+Pasteur.langEN – PDF
J. Gal (2008) The discovery of biological enantioselectivity: Louis Pasteur and the fermentation of tartaric acid, 1857—A review and analysis 150yr later Chirality: 20: 5-19. https://doi.org/10.1002/chir.20494 https://onlinelibrary.wiley.com/doi/10.1002/chir.20494
J. Gal. (2017) Pasteur and the art of chirality. Nature Chem 9, 604–605. https://doi.org/10.1038/nchem.2790
Y. Tobe (2003) The re-examination of Pasteur’s experiment in Japan. Mendeleev Communications Electronic Version Issue 3. 93-94 Royal Society of Chemistry. https://pubs.rsc.org/en/content/articlelanding/2003/mc/x0013093
X.Wang et al. (2012) How To Crystallize Anhydrous Racemic Tartaric Acid from an Ethanol–Water Solution Industrial & Engineering Chemistry Research 51(6):2789–2796 DOI:10.1021/ie201935t
Science History Institute (2017) Louis Pasteur.
https://www.sciencehistory.org/historical-profile/louis-pasteur
J.A. Considine, L. Dip, and E. Frankish (2014) A Complete Guide to Quality in Small-Scale Wine Making. Academic Press (Elsevier) England. 214 pg. Downlad PDF or Purchase book.
P. Ribereau-Gyon, Y. Glories, A. Maujean and D. Dubourdieu (2006) Handbook of Enology Vol 2 The Chemistry of Wine Stabilization and Treatments 2nd Edition Wiley & Sons Ltd. PDF
J. Cuber (2020) History of Wine Making - https://storymaps.arcgis.com/stories/c5a01856223745d19ee5a2f640624b83
Brown, William H.. "Tartaric acid". Encyclopedia Britannica, 17 Apr. 2016, https://www.britannica.com/science/tartaric-acid
Wassily Kandinsky – Wikipedia https://en.wikipedia.org/wiki/Wassily_Kandinsky
C. Butzke Wine Cold Stability Issues. Purdue Extension - PDF
R. Berdan (2017) The Art & Science of Photomicrography with Polarized light.
R. Berdan (2019) Crystals Photographed with Polarization Microscopy: Water, Beer, Caffeine, Vitamins, Amino Acids and Human Tears.
R. Berdan (2021) Curved Crystals viewed by Polarized Light Microscopy Amino acids, Vitamin C, Menthol & Caffeine.
R. Berdan (2021) Polarization Microscopy The Motic BA310 Polarizing Microscope a Review
R. Berdan (2022) Photomicrography of Crystals with a Polarizing Microscope for Art’s Sake - Amino acids, Cannabis (THC) and Caffeine
R. Berdan (2022) Crystals by Polarized Light Microscopy Science & Art Part II
Zeiss (2023) Vitamin C Crystals - The Magic of Polarized light Microscopy featuring R. Berdan
Wine Art
Suzzane Groet - Tartaric acid crystals Niikon 1979 photomicrography contest.
Marek Mis - Tartaric acid crystals as art
Loes Modderman - Tartaric Acid crystals on Molecular Expressions
Amy Giacomelli - Abstract Wine Art ... Whites and Reds - https://pixels.com/featured/abstract-wine-art--whites-and-reds-amy-giacomelli.html
Natiliemaclean Wine art on bottles - https://www.nataliemaclean.com/blog/wine-label-art-design/
Pininterest - Wine Art - https://www.pinterest.ca/pin/139470919685995672/

Wine cork art by an unknown artist from Bragg Creek, AB.
Authors Biography & Contact Information
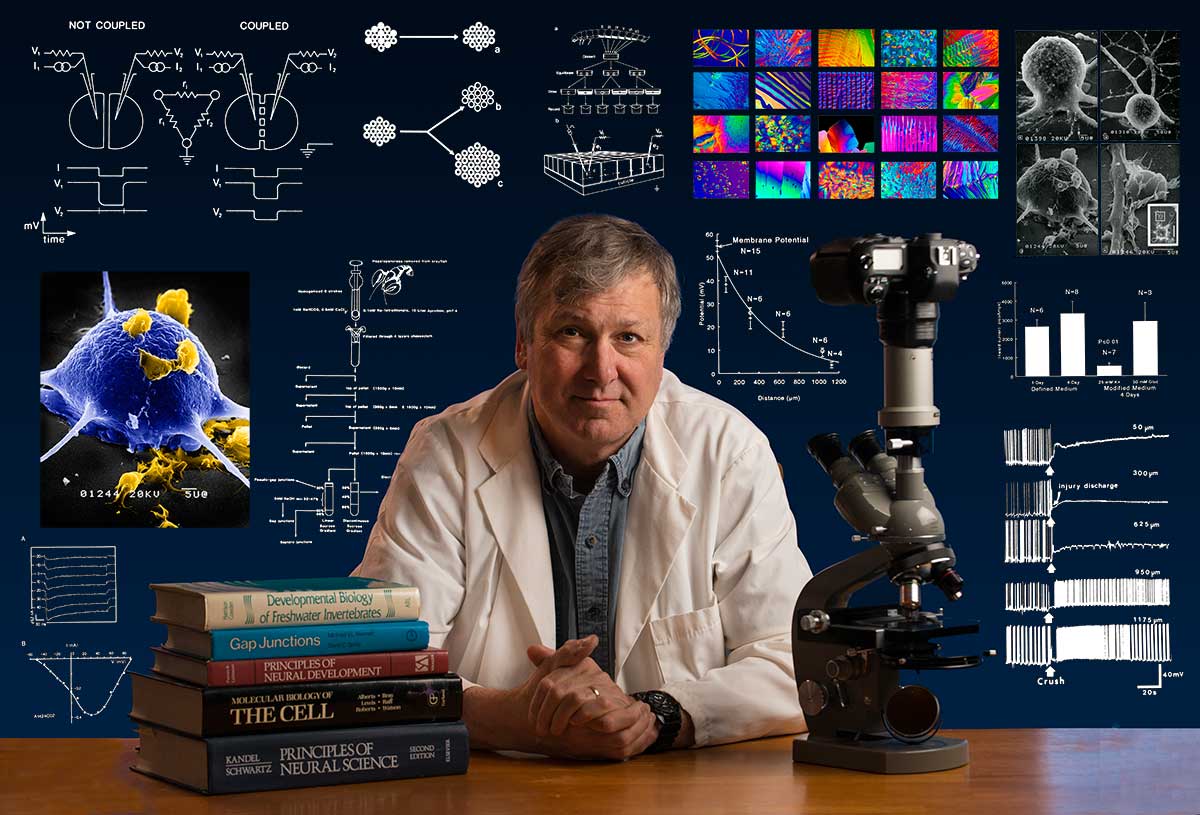
Bio: Robert Berdan is a professional nature photographer living in Calgary, AB specializing in nature, wildlife and science photography. Robert retired from Cell\Neurobiology research to pursue photography full time many years ago. Robert offers photo guiding and private instruction in all aspects of nature photography, Adobe Photoshop training, photomicrography and macro-photography. Portrait of Robert by Dr. Sharif Galal showing some examples of Robert's science research in the background.
Email at: rberdan@scienceandart.org
Web sites: www.canadiannaturephotographer.com
www.scienceandart.org
Phone: MST 9 am -7 pm (403) 247-2457.